
Fact Sheets And Publications

The Impacts of Nitrogen and Phosphorus from Agriculture on Delaware’s Water Quality
May 2025 | Written by: Jennifer Volk and Amy L. Shober
Introduction
Nitrogen (N) and phosphorus (P) are essential nutrients for all living organisms. Soil, fertilizer, and manure are all sources of N and P to growing crops. Atmospheric deposition (the air we breathe is mostly N gas) and irrigation water are also N sources. If not managed efficiently, much of the N and P applied to and present in agricultural systems can be lost to the environment. If N or P are present in aquatic systems (streams, ponds, estuaries, etc.) above critical levels, negative environmental impacts occur.
Causes of Nitrogen and Phosphorus Loss from Agricultural Fields
Nitrogen Loss
Nitrogen exists in several forms, both organic and inorganic. The two main forms of inorganic (plant available) N are nitrate (NO3-) and ammonium (NH4+). There are three different processes to describe how N can be converted from one form to another.
Denitrification: Nitrate is turned into N gas under wet conditions.
Volatilization: Depending on a variety of factors including type and placement of fertilizer and certain soil conditions, ammonium can be converted to ammonia gas.
In both denitrification and volatilization, the gases escape to the atmosphere where the N is no longer available to plants.
Mineralization: Organic forms of N can be converted to the inorganic forms by bacteria in the soils making the N available to plants. This also occurs more rapidly under warm and moist conditions.
Mineralized N on agricultural lands may also be lost to the aquatic environment, which has significant impacts on the ecosystem. Sediment bound N can be eroded by wind and water then carried to streams and rivers suspended in runoff. More often though, N contained in soil, crop residues, fertilizers, and manures dissolves in water. While some of the dissolved N also leaves in runoff, the majority leaches through the soil past the root zone in subsurface drainage. Dissolved N leaves through natural underground laterally flowing water, or through artificial drainage systems like tile drains or drainage ditches (Figure 1).
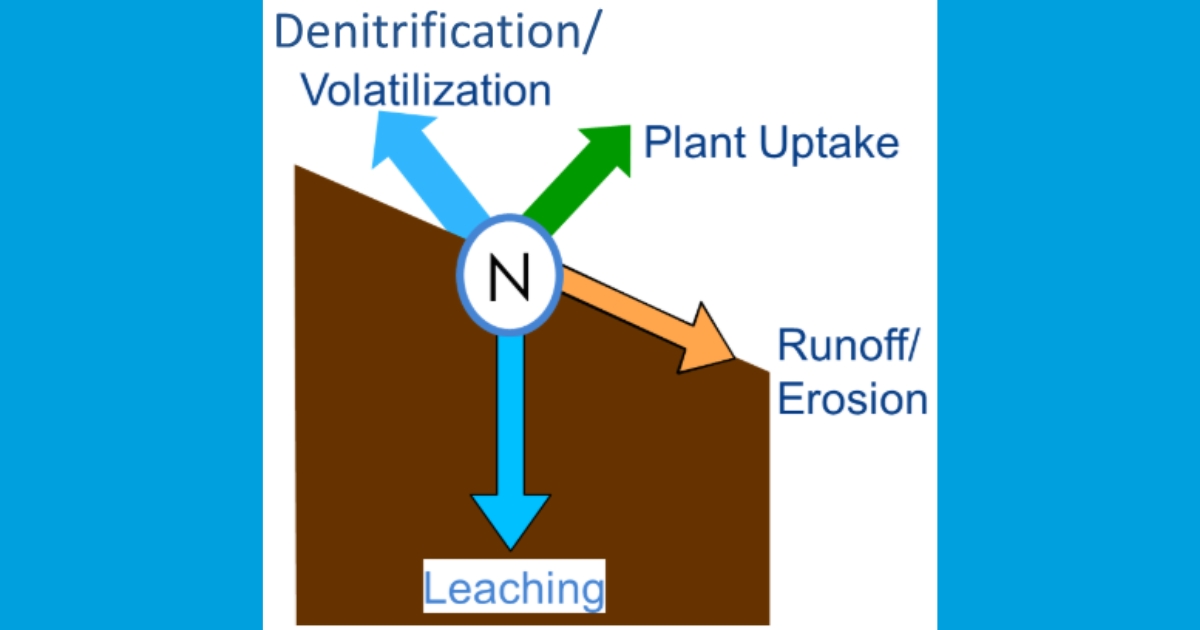
Phosphorus Loss
The potential for loss of P from agricultural lands is dependent on several factors. They are often grouped into two categories: source factors and transport factors.
Source factors include soil P levels, P application practices (source of nutrient, such as manure or commercial fertilizer; method; amount; rate; and timing), and field management practices like tillage and use of cover crops.
Transport factors include the potential for loss through erosion, surface runoff, seepage, or subsurface drainage and the proximity to bodies of water.
Sediment bound P loss through erosion: Phosphorus often attaches to the surface of soil or organic matter particles. Erosion of agricultural soils by wind or water can move this sediment bound (or particulate) P in surface runoff. Particulate P becomes suspended in runoff water and can then be transported to the nearest body of water. Phosphorus loss caused by erosion is greatest from plowed fields where the land is sloped and the soils are mostly finer textured (e.g., silty or clayey).
Dissolved P loss through water: Phosphorus can also be lost from agricultural fields when it becomes dissolved in water. The potential for which is often greatest in fields managed under conservation tillage or no-till because there is less soil disturbance. During a rain event, a portion of P contained in soils, crop residues, fertilizers, and manures can dissolve. Like N, this dissolved P can be lost from agricultural fields in surface runoff. Dissolved P can also move through the soil and be lost below the root zone in subsurface drainage. Phosphorus can move through natural underground laterally flowing water, or through artificial drainage systems like tile drains or drainage ditches. As dissolved P moves along the soil surface or percolates into soil, it may become reattached to soil particles (Figure 2).

Impacts of Nitrogen and Phosphorus on the Aquatic Environment
Nitrogen and P are also essential elements in the aquatic environment. However, agricultural and urban land practices add more N and P to surface waters than they would receive under natural conditions. When one or both of these nutrients exceed critical concentrations, pollution of downstream water bodies can occur. High concentrations of nutrients in water bodies fuels the overgrowth of algae which impacts the local ecology by blocking sunlight from reaching beneficial submerged aquatic vegetation.
Nutrient pollution often leads to large daily fluctuations in dissolved oxygen levels. Dissolved oxygen is used by fish and shellfish to breathe. Organisms that can escape the potentially lethal low dissolved oxygen levels leave, while those organisms that cannot leave typically die. Thus, nutrient pollution is often associated with fish and shellfish kills. As water quality declines, an ecosystem’s species diversity and abundance also decline. Other negative characteristics of nutrient pollution include poor water clarity, foul odors, insect problems, and shallow depths that impede water flow and boat navigation.
The Delaware Department of Natural Resources and Environmental Control (DNREC) has established nutrient criteria to establish the health of the ecosystem for portions of Delaware’s Inland Bays, which are an important estuarine habitat. During the growing season for aquatic vegetation (approximately March 1 - October 31), the tidal portions of Indian River Bay, Rehoboth Bay, and Little Assawoman Bay must have average dissolved inorganic N concentrations less than 0.14 milligrams per liter (mg/L) and average dissolved inorganic P concentrations less than 0.01 mg/L to be considered healthy (7 DE Admin. Code § 7401).
For the freshwater streams within Delaware, concentration threshold values are utilized to assess if a waterway is impaired by nutrients. The threshold for N is 3.0 mg/L total N. For P, the threshold is between 0.1 and 0.2 mg/L total P. When nutrient concentrations are observed above these values, other symptoms of nutrient pollution, especially low dissolved oxygen concentrations, are also often present.
Delaware’s Water Resources
Delaware has four major basins (drainage areas), which define where waters flow:
Piedmont Drainage is in northern Delaware;
Delaware Bay Drainage consists of most of the central eastern part of the state;
Chesapeake Bay Drainage includes the western portion of the state; and
Inland Bays/Atlantic Ocean Drainage is located in southeastern Delaware.
These four larger basins can be subdivided into 45 smaller watersheds associated with individual stream or river networks. Watersheds are the land areas that drain to a specific body of water.
Streams (surface water) are fed by the water flowing over the land when it rains (runoff), as well as the water that soaks through the soil into underground aquifers (groundwater). In some central and southern Delaware watersheds, groundwater makes up most of the water in the streams on an annual average basis. Thus, ground and surface waters are closely linked and the reason why our actions on the land impact the health of our waters.
Clean water is not only important for aquatic plants and animals, but also very important for human health. In northern Delaware (above the C&D Canal) drinking water is primarily provided by surface waters, while below the canal, drinking water is derived from groundwater sources. The national drinking water standard for N is 10 mg/L nitrate. There is no national drinking water standard for P. When concentrations exceed this level of N, there is risk for a condition known as methemoglobinemia, or blue baby syndrome, because the excess nitrate limits the oxygen that can be carried by the blood.
Laws and Regulations Addressing the Environmental Impacts of Nitrogen and Phosphorus
Decades of ground and surface water monitoring have revealed water quality concerns. High levels of nitrate have been measured in groundwater (18% of sampled domestic wells across the state exceeded the drinking water standard; Pellerito et al., 2008) and polluted conditions have been observed in many of Delaware’s surface waters. Water quality monitoring of Delaware’s streams, ponds, and bays has confirmed that oxygen levels are often too low to fully support aquatic life and that nutrient concentrations are higher than they would be under natural conditions. As a result, many of Delaware’s surface waters have been classified as impaired and limits have been placed on the amounts of N and P that can enter each waterway (DNREC, 2022).
These limits are regulations called Total Maximum Daily Loads (TMDLs). Each TMDL addresses the nutrient reductions necessary from point sources, such as wastewater treatment plants, and nonpoint sources, such as agriculture and urban land uses. In Delaware, the TMDL nonpoint reductions required range from 0–85% for N and 0–65% for P. Plans or strategies for many of Delaware’s watersheds that have high nutrient levels have been developed and describe how these levels should be reduced. For more information about TMDLs, refer to Watersheds: Understanding Total Maximum Daily Loads (TMDLs) (available at http://www.udel.edu/0013545).
In 1999, the Delaware legislature adopted the Delaware Nutrient Management Law (3 Del. C. §§ 2201-2290). The purpose of the law is “to regulate those activities involving the generation and application of nutrients in order to help improve and maintain the quality of Delaware's ground and surface waters…” The law requires certification and nutrient management plans for most agricultural operations with 8 or more animal units or that apply nutrients to 10 or more acres.
Additionally, nutrient management regulations (3 DE Admin. Code §§ 1201-1204) prohibit the application of both commercial and manure based fertilizers between December 7th and February 15th and to snow covered or frozen ground since this is when nutrients are most vulnerable to runoff. The law also limits the application of P to “high” P soils to a rotational crop removal rate unless higher rates are approved following a field level risk assessment using the Delaware P Site Index. Nutrient storage and staging requirements for manure are also specified in the regulations to minimize losses to the environment. For more information about the requirements outlined in the Delaware Nutrient Management Law and Regulations, refer to Understanding the Requirements of the Delaware Nutrient Management Law (available at http://www.udel.edu/0013546).
Nitrogen and Phosphorus Management Strategies for Agriculture
Since many of Delaware’s surface waters have TMDL regulations that require reductions of nonpoint N and P, it is important for Delaware’s agricultural producers to consider and implement various nutrient management strategies. These strategies will not only help to protect Delaware’s ground and surface waters from pollution and achieve water quality goals, but could also help agricultural producers become more efficient and profitable. Additionally, cost-share programs are available from the US Department of Agriculture, Delaware Department of Agriculture, and the New Castle, Kent, and Sussex Conservation Districts to help support the adoption and implementation of many of these strategies.
A nutrient budget will balance inputs from the various sources and forms of N and P with the outputs to minimize excess available for loss and will take into account crop N removal rates and crop P removal rates. Fertilizer application strategies that consider the amount, form, timing, and method of application will also minimize losses of N and P from agricultural soils. Additionally, tillage, cropping, and conservation practices can also be instituted to help trap and remove excess N and P.
Nitrogen Strategies
Fertilizer application strategies for N, may include utilizing a split application approach in order to increase N use efficiency by applying a small amount at planting and the remainder when growth is active. Alternatively, fertigation is possible for those producers utilizing irrigation. Fertigation is the application of fertilizers (including the nutrients already available in groundwater sources) as needed through the irrigation system. Several tools and tests, like the pre-sidedress soil nitrate test for corn (PSNT) and the leaf chlorophyll meter, are available to help determine the appropriate amount of N to apply and when N is needed. For more information about the PSNT, refer to Nitrogen Management for Corn in Delaware: The Pre-sidedress Nitrate Test (available at http://www.udel.edu/0013425). Additionally, the end-of-season corn stalk nitrate test can be used to assess if N had been under or over-applied during the growing season. For more information about the corn stalk nitrate test, refer to End-of-season Corn Stalk Nitrate Testing to Optimize Nitrogen Management (available at http://www.udel.edu/0013391).
Phosphorus Strategies
Prior to applying P to crop fields a soil test should be completed to determine how much P will be available to crops from the soils. For more information about the soil testing for P, refer to Interpreting Soil Phosphorus and Potassium Tests (available at http://www.udel.edu/0013426). Additionally, the P Site Index should be used if the soil test value for P is greater than 150 FIV (Fertility Index Value reporting unit; equivalent to 150 mg/kg Mehlich-3 P) to assess the risk of P loss from agricultural fields. Then, based on the P Site Index results, soil management options can be considered. For more information about the P Site Index and management options based on the P Site Index, refer to Phosphorus Management Strategies for Delaware’s Agricultural Soils: The Phosphorus Site Index (available at http://www.udel.edu/0013425) and Soil Management Options based on the Phosphorus Site Index (available at http://www.udel.edu/0013355).
Summary
While N and P are necessary nutrients in both the agronomic and aquatic environments, excessive losses of these nutrients from agricultural fields, suburban, and urban settings can negatively impact our water resources. Regulatory measures have been taken to reduce the amount of N and P that reach many of Delaware’s waterways in order to protect aquatic life and human uses like drinking water. Agricultural operations can assist in achieving these goals by employing a number of nutrient management strategies that not only reduce N and P losses to the environment but may also improve the overall profitability of the operation through the more efficient use of fertilizers.
References
Delaware Department of Natural Resources and Environmental Control (DNREC). (2022). State of Delaware 2022 combined watershed assessment report (305(b) and determination for the Clean Water Act Section 303(d) list of waters needing TMDLs. Delaware Department of Natural Resources and Environmental Control. https://documents.dnrec.delaware.gov/Watershed/Assessment/Reports/2022-Delaware-Final-IR-with-appendices.pdf
Nutrient Management, Delaware Administrative Code Title 3 Chapter 22. (3 Del. C. §§ 2201-2290). https://delcode.delaware.gov/title3/c022/index.html
Nutrient Management Certification Regulations, _D.E. Administrative Code, Title 3, § 1201. (3 DE Admin. Code 1201). https://regulations.delaware.gov/AdminCode/title3/1200/1201.shtml#P2_24
Pellerito, V., Neimeister, M. P., Wolff, E., & Andres, A. S. (2008). Open File Report No. 48: Results of the domestic well water-quality study. Delaware Geological Survey, University of Delaware. https://www.dgs.udel.edu/sites/default/files/publications/OFR48.pdf
Surface Water Quality Standards, Delaware Administrative Code Title 7 Section 7401. (7 DE Admin. Code § 7401). https://regulations.delaware.gov/AdminCode/title7/7000/7400/7401.shtml#TopOfPage
About the Authors
Jennifer Volk, Associate Director, University of Delaware Cooperative Extension, Dover, DE
Amy L. Shober (corresponding author), Professor and Extension Specialist, University of Delaware, Newark, DE (ashober@udel.edu)
About this Publication
Original Publication Date: July 2013
Revision date(s): 2025
Peer Reviewers
Dr. Robin Tyler. Delaware Department of Natural Resources and Environmental Control. Dover, DE.
UD Cooperative Extension
This institution is an equal opportunity provider.
In accordance with Federal law and U.S. Department of Agriculture policy, Cooperative Extension is prohibited from discriminating on the basis of race, color, national origin, sex, age, or disability.
