
Fact Sheets And Publications

Anthracnose Leaf Blight and Stalk Rot of Corn
Anthracnose leaf blight and stalk rot of corn, caused by the fungus Colletotrichum graminicola, is a disease of worldwide importance. Yield losses can approach 40% and up to 80% lodging has been observed in fields with severe levels of anthracnose. Anthracnose can be found in corn produced in Delaware and can pose problems to local growers. This publication will go over disease identification, the disease cycle, and management options.
Disease Identification
Anthracnose can be found on all parts of corn throughout the growing season. However, the disease is most often observed as a 1) leaf blight or spot, 2) top dieback, or 3) stalk rot.
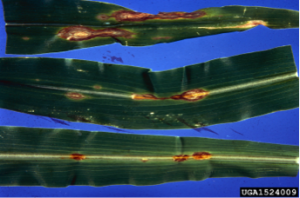
- Foliar symptoms of anthracnose may be observed early in the season on young seedlings (prior to V6). On foliage the fungus produces small, oval to irregular, brown to red-brown lesions, often with a yellow margin (Figure 1a). Lesions often have a “target-like” appearance due to concentric growth of the fungus. Fungal bodies surrounded by black hairs may be observed within lesions with the use of a 20-30x hand lens, and spores are observed with the use of a microscope (Figure 1b). Foliar symptoms closely resemble those of other foliar diseases of corn, making field diagnosis difficult. After the V6 stage the foliar phase of the disease can be greatly reduced in some hybrids because of the accumulation of defensive chemicals in leaves. A second flush of foliar symptoms may be noticed on upper foliage after tasseling in some hybrids. Reductions in yield are likely if leaf blighting occurs before six weeks after tasseling.

2) With the aid of a hand lens or microscope fungal structures can be observed. The most conspicuous feature is the presence of black structures with spines, which are characteristic of C. graminicola.
3) Top dieback caused by the anthracnose pathogen is characterized by yellowed, purple, or dead/dying flag leaves on plants scattered throughout the field. When the leaf sheaths are peeled back at the top of the affected plants, shiny black lesions can be observed on the outside of the stalk. Under appropriate conditions a salmon-colored gel can be seen on the stalk. This gel contains spores of the fungus. If the stalk of the top is split, the pith will often appear discolored and rotted in the upper internodes.
Symptoms of anthracnose stalk rot are easy to identify. Late in the growing season plants will senesce prematurely. On these plants black, shiny streaks or spots can be observed on the lower portions of the stalk. In some cases the entire stalk may become black. Affected plants will fall when pushed and stalks can be squeezed easily between the thumb and forefinger. When the stalk is split, the pith will appear discolored and rotted as in the top dieback phase. Lodging can occur in plants infected with stalk rot.
Disease Cycle
C. graminicola overwinters in corn residue on the surface of the field. In the spring, under warm, rainy conditions the fungus produces spores on residue, which can infect roots and foliage of corn seedlings. If infection occurs below the soil surface the root and pith are colonized and disease may not be observed until later in the season. Spores that are rain-splashed to seedlings cause primary infections. If the environment remains warm, rainy, and overcast, spores can be continually produced on foliar lesions resulting in severe foliar symptoms on some hybrids. Continued spore production may result in disease spread up the plant after tasseling. If the plant is mechanically damaged (e.g. hail, insects) infections of the leaf sheath may spread to the stalk. As the plant reaches maturity C. graminicola located within the stalk begins to decompose the pith for nutrients, greatly reducing stalk integrity and resulting in premature plant death [4].
Management
Management for anthracnose is best achieved by resistant hybrids, residue management, crop rotation, and stress mitigation. Crop losses are often a result of lodging due to systemic stalk infections, which are more likely to originate from root infections. Consequently fungicides are not recommended in Delaware for control of anthracnose stalk rot. Scouting is important for mitigating impacts of anthracnose on corn. Fields should be scouted for stalk rots a few weeks before corn approaches maturity. Ten sections of the field should be scouted for stalk rot. If the field has greater than 10% incidence of stalk rot, the field should be scheduled for early harvest to mitigate effects of lodging-associated yield loss.
Resistant hybrids
Many hybrids with good resistance to anthracnose stalk rot are commercially available. Resistance to other stalk rots such as those caused by Gibberella spp. or Diplodia spp. may not carry over to anthracnose stalk rot. Confirmation of the disease by sending samples to your local diagnostic clinic is useful in making hybrid selections. Select hybrids with good stalk strength and stay green ratings.
Residue management
Practices that favor the decomposition of infested corn tissues will reduce inoculum to cause disease in subsequent years. Disease severity is greatest in no-till situations. Tillage, if practical, prohibits the production and dissemination of spores and enhances tissue decomposition. In Delaware, chopping, shredding, or disking fields after harvest may aid in decomposition of corn residue.
Crop rotation
C. graminicola overwinters well on corn tissues, as well as other grasses and small grains. Rotation with a non-grass crop, such as soybean, can help reduce effects of this disease in the subsequent corn crop. Avoid corn-after-corn rotations, particularly if the field has a history of anthracnose.
Mitigate plant stress
Severity of stalk rots increase with plant stress. Growers should avoid excessive water and water stress, utilize a balanced nutrient management program. Insect pressure and foliar diseases can reduce photosynthetic area, resulting in the plant remobilizing an excessive quantity of carbohydrates to the ear during grain fill. When this occurs the stalks are more susceptible to stalk rots, lodging, and yield losses.
References
1. Lipps, P.E., Survival of Colletotrichum graminicola in infested corn residues in Ohio. Plant Disease, 1983. 67(1): p. 102-104.
2. Keller, N.J. and G.C. Bergstrom, Wound predisposition of corn stalks to Colletotrichum graminicola. Phytopathology, 1983. 73(9): p. 1344-1344.
3. Bergstrom, G.C., B.S. Croskey, and R.I. Carruthers, Synergism between Colletotrichum graminicola and European corn borer in stalk rot of corn in New York. Phytopathology, 1983. 73(5): p. 842-842.
4. Venard, C. and L. Vaillancourt, Growth of and colonization by Colletotrichum graminicola inside corn stalk tissues. Phytopathology, 2005. 95(6): p. S107-S107.
Date Published: 02/2014
Author(s): Nathan Kleczewski Ph.D.
Extension Plant Pathologist
UD Cooperative Extension
This institution is an equal opportunity provider.
In accordance with Federal law and U.S. Department of Agriculture policy, Cooperative Extension is prohibited from discriminating on the basis of race, color, national origin, sex, age, or disability.
