
Liming Materials and Management

A Comparison of Methods to Determine Lime Requirement
June 2025 | Written by: Amy L. Shober, Karen L. Gartley, and J. Thomas Sims
Introduction
Managing soil pH is crucial for maximizing crop yields and promoting overall soil health. The optimal soil pH is typically between 5.5 and 7.0 for most crops growing in Delaware. Maintaining a desirable soil pH ensures that essential nutrients are readily available for plant uptake and minimizes the potential for toxicity from elements like aluminum (Al) and manganese (Mn), which can become harmful in overly acidic conditions. Furthermore, proper calculation of liming rates needed to maintain soil pH prevents over-liming, which can make soils too alkaline and result in nutrient deficiencies. Delaware soils are generally at risk for over-liming due to their sandy texture and relatively low cation exchange capacity (CEC). Finally, proper pH management plays a vital role in preventing the accumulation of non-essential trace elements in the food chain when certain soil amendments are applied.
The comprehensive management of soil pH involves several key steps: (1) selection of the target pH for the specific crop and soil type; (2) determining the current soil pH in water or weak salt solution; (3) determining the base lime requirement using an appropriate lime requirement test; (4) adjusting lime rates based on previous lime applications and lime source; and (5) selection and application of a liming material. The purpose of this fact sheet is to describe the various lime requirement tests and the associated equations that are used by regional soil testing laboratories to calculate the base lime requirements to achieve optimal soil pH for production of crops in Delaware (i.e., step 3 in management of soil pH). For more information about the other steps involved in managing soil pH, refer to Measurement and Management of Soil pH for Crop Production in Delaware (available at http://www.udel.edu/0013466) and Liming Materials and Management (available at http://www.udel.edu/0013552 ).
What is a Lime Requirement Test?
When soil pH falls below the desired target, a lime requirement test becomes essential. The lime requirement test determines the amount of agricultural limestone (or other liming material) needed to raise the soil pH from an acidic state to a level that is optimal for crop production. This "lime requirement" may also be referred to as the capacity factor of soil acidity because it reflects the total soil acidity that needs to be neutralized.
Total soil acidity has two main components:
Active acidity refers to the hydrogen (H+) ions found in the soil solution.
Reserve acidity includes hydrogen ions bound to soil particles, as well as acidity contributed by soil aluminum (Al) and iron (Fe). This reserve acidity is what gives soil its buffering capacity—its ability to resist changes in pH.
The primary goal of liming is to neutralize enough of this total acidity, usually within the "plow layer" (the top layer of soil), to reach the desired pH.
A routine soil pH test (using a water or weak salt slurry method) only measures active acidity and does not account for reserve acidity. As such, relying on the routine soil pH test alone for lime recommendations can severely under- or over-estimate the amount of lime needed to neutralize acidity. Therefore, a lime requirement test should be performed on any soil with a routine soil pH test that is below the target pH for the crop to be grown.
Principles of Lime Requirement Testing
Since effective soil management requires adjusting soil pH to an acceptable range, accurate and fast methods for determining lime needs are vital. Many tests are available, and excellent reviews on the topic exist (Alley & Zelazny, 1987; Sims, 1996; van Lierop, 1990). All lime requirement methods, however, follow similar fundamental principles:
The measured lime requirement must accurately reflect the actual amount of liming material needed to raise the soil pH to the desired target under field conditions.
Lime requirement tests must precisely measure all forms of acidity present in a soil—both active and reserve—that need to be neutralized.
The test must be backed by comprehensive calibration studies that consider the soil's intended use and the expected variation in soil properties within the geographic area where the test will be applied.
Factors Influencing Lime Requirement
Several key factors determine how much lime your soil needs. Understanding these will help you interpret lime recommendations.
Nature of soil acidity: The amount of lime required depends on the source of soil acidity. Acidity can be due to hydrogen ions (H+) in the soil solution, or H+ released from soil clays, organic matter, and aluminum (Al) and iron (Fe) oxides. Exchangeable Al+3 is a significant contributor of acidity, as are H+ additions from acid rain, reactions of acid forming fertilizers, and mineral weathering.
Current vs. target pH: The amount of lime needed is directly related to how far the soil pH is from its desired target pH. As you add lime and the pH increases, the forms of acidity in the soil change. For example, in very acidic soils (pH < 5.0), lime primarily neutralizes exchangeable Al+3. As the pH approaches 5.5, aluminum hydroxides and soil organic matter become the main sources of acidity. Accurate lime requirement tests must account for all these forms of acidity.
Soil Properties (Buffering Capacity): A soil's buffer capacity—its ability to resist changes in pH—greatly influences how much lime is needed. Soils with more clay, Al and Fe oxides, and organic matter have a higher buffer capacity, meaning they require more lime to achieve the same pH change as poorly buffered sandy soils. Sandy soils need less lime because they tend to be poorly buffered, however, they are also more susceptible to over-liming, which can lead to micronutrient deficiencies in plants.
Comparing Lime Requirement Methods
Soil testing laboratories employ various techniques to estimate the lime requirement of acidic soils, with the most appropriate method depending on the specific soil characteristics and region. Generally, these methods fall into a few categories:
Soil Buffer pH Tests
Soil buffer pH tests are the most common methods for routine soil testing. The buffer pH tests involve adding a chemical solution that is strongly buffered at a high pH (typically 7.5-8.0) to a soil sample. After a brief equilibration, the pH of the mixture is measured. The decrease in the buffer's pH is directly proportional to the soil acidity that needs neutralizing. A variety of buffer solutions are available, each designed for different soil types:
Adams-Evans Buffer: This buffer was specifically developed for the coarse-textured, poorly buffered soils of the Atlantic Coastal Plain (e.g., Delaware and parts of the Mid-Atlantic). This buffer is excellent at detecting subtle changes in lime requirement, helping prevent over-liming and associated micronutrient deficiencies. The University of Delaware Soil Testing Laboratory uses the Adams-Evans buffer test to determine base lime requirements.
Mehlich Buffer: The Mehlich buffer is also suitable for coarse-textured soils with low cation exchange capacity (CEC) and buffering capacity.
Shoemaker-Mclean-Pratt (SMP)/Sikora Buffer: These buffers are designed for heavier soils with higher CEC and lime requirements, common in the Northeastern and North Central U.S.
For a list of soil buffer pH tests offered by labs in the Mid-Atlantic, refer to Measurement and Management of Soil pH for Crop Production in Delaware (available at http://www.udel.edu/0013466). It is important to note that other buffer tests (e.g., Woodruff, Sikora-2) are used in other regions of the U.S. Plus, because some buffer pH tests (including Adams-Evans, SMP, and Mehlich buffer) utilize hazardous chemicals, researchers have recently developed modifications to these tests to incorporate more environmentally friendly chemicals. Consequently, researchers have developed modifications to these tests to incorporate more environmentally friendly chemicals, and these newer versions have been adopted by some laboratories. Examples include the Sikora buffer (a modification of the SMP test), Moore-Sikora and modified Adams-Evans (modifications of the Adams-Evans test), and a modified Mehlich buffer that replaces the traditional version. Using the correct buffer for the specific soil type is crucial, as using an inappropriate buffer can lead to significant over- or under-estimations of lime needs. Under liming can result in wasted time and money, while over liming can result in reduced availability of micronutrients, increased volatilization of ammonium-based fertilizers, and reduced P availability.
Direct Measurements of Exchangeable Acidity (or Aluminum)
Soil testing laboratories in the U.S. rarely make direct measurements of exchangeable acidity or Al for routine recommendations due to the higher time and expense involved in handling and analyzing a separate sample. This method involves extracting exchangeable aluminum (Al) with a neutral salt solution (like potassium chloride) and then titrating with a standard base. For highly weathered, acidic mineral soils where toxic levels of exchangeable Al are the primary cause of infertility, neutralizing this Al can eliminate acidity as a yield-limiting factor. This approach often results in a lower target pH (around 5.5-5.8) compared to buffer pH methods.
Estimates of Lime Requirement Based on Soil pH and Soil Properties
Sometimes lime requirements are estimated using existing soil pH data combined with other measured or recorded soil properties that relate to buffer capacity, such as organic matter content, estimated soil texture, or soil series. For example, Maryland's lime recommendations consider soil pH and texture. While this approach can be reasonably accurate, it is less common than buffer pH tests because it requires either detailed soil type information or an additional measurement on the same soil sample (e.g., soil texture or organic matter analysis).
Automated Soil Titrations
These methods directly measure a soil's buffering capacity by adding a base and observing the resulting pH change. For instance, the University of Georgia uses a single-point automatic soil titration method with calcium hydroxide [Ca(OH)2]. In this procedure, a base like Ca(OH)2 is added to a soil sample, and the change in pH is used to calculate the lime buffer capacity. While this method can be highly accurate and offers advantages in terms of direct measurement of buffering, few laboratories routinely offer it due to the complexity and specialized equipment needs involved in automated titrations. These methods are more commonly found in research settings.
Ultimately, the most appropriate lime requirement test depends on the specific characteristics of the soils in your geographic area. Years of research and practical experience have shown that different tests yield the most accurate recommendations in various physiographic regions. Recent recognition of discrepancies in lime requirement recommendations across the U.S. (even when using similar lime requirement methods) has sparked renewed interest in recalibration of lime requirement tests. Researchers across the U.S. are actively working to improve lime recommendations using diverse methodologies, with vital work ongoing at a national scale as part of National Research Project NSRP-11, Building Collaborative Research Networks to Advance the Science of Soil Fertility: Fertilizer Recommendation Support Tool (FRST).
Equations Used to Calculate Lime Requirement
Base lime recommendations can be calculated for each lime requirement test method using specific equations that were derived from calibration studies using that test method. Due to the complexity of the equations, we provide simplified look up tables that provide base lime rates as a function of soil pH and buffer pH for the main lime requirement tests that are used by regional soil testing laboratories in Measurement and Management of Soil pH for Crop Production in Delaware (available at http://www.udel.edu/0013466). These tables provided the lime rate (tons/ac) for each target pH group based on the water pH and buffer pH, both of which are typically included on soil test reports. The tables assume the use of agricultural grade calcitic (“Hi-Cal’) or dolomitic (“Hi-Mag”) liming materials, which are estimated to have neutralizing power that is 67% as effective as pure CaCO3 (e.g., 67% effective calcium carbonate content [ECCC]). Information about other liming materials and how to calculate ECCC is available in the document Liming Materials and Management (available at http://www.udel.edu/0013552). To adjust base lime rates from the look up tables for alternative liming materials, use the following equation:
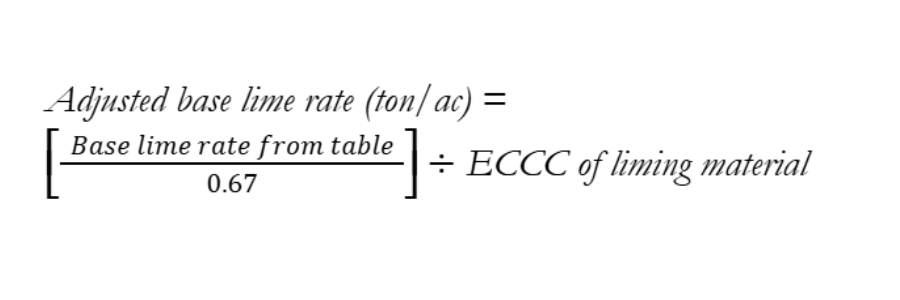
Where ECCC is expressed as a decimal (e.g., 0.67 = 67% ECCC).
Here we provide the detailed lime requirement equations for those who prefer to calculate lime requirements directly. The lime recommendation equations for the Adams-Evans buffer are the only equations in this document that were based on calibration studies conducted in Delaware (20 soil types from Delaware; Sims & Dennis, 1988). Other lime recommendations equations are based on calibration studies that occurred in other locations.
Adams Evans pH
The base liming rate is calculated from the target pH, water pH and buffer pH using the equations:
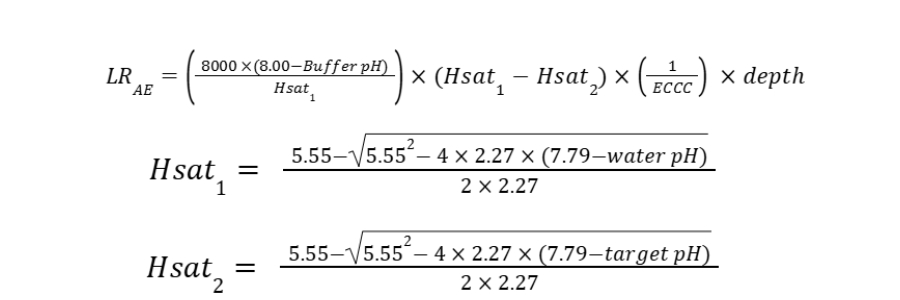
Where:
LRAE= lime rate in lbs/ac of liming material (divide this value by 2000 to convert to ton/ac)
Hsat1 = acid saturation of the soil at the current soil pH
Hsat2 = acid saturation of the soil at the target pH
Water pH = the soil pH measured in a soil-water slurry with a 1:1 soil to solution ratio
Buffer pH = Adams-Evans buffer pH obtained from the soil test report
ECCC = effective calcium carbonate equivalent expressed as a decimal (e.g., 0.67 for agricultural-grade limestone with 67% ECCC)
Depth = correction factor for soil sample depth as compared to a 6.67-inch furrow slice (e.g., use 0.90 for a 6-inch sample and 1.18 for an 8-inch sample).
Mehlich and Modified Mehlich Buffer
Some regional laboratories (i.e., Penn State, Virginia Tech) base their lime recommendations on soil Mehlich buffer pH and soil type (e.g., mineral soil vs. soils high in organic matter).
We recommend the lime recommendation calculations that are based on both the Mehlich buffer pH and soil pH (1:1 soil to water) as described by Hardy et al. (2014), which is similar to how we calculate lime requirement using the Adams-Evans Buffer test. The Mehlich buffer pH is used to calculate buffer pH acidity (AC) using the following equation:
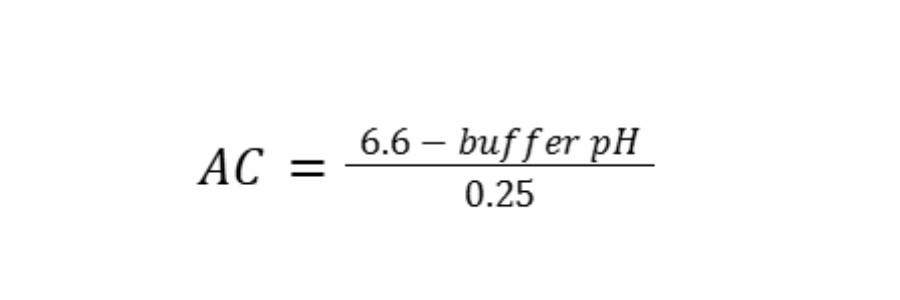
Where:
AC = acidity expressed in units of meq/100 cm3
Buffer pH = Mehlich or Modified Mehlich buffer pH.
The lime requirement (tons/ac) for a 90% ECCC material is then calculated using the following equation:

Where:
LRM = lime rate (ton/ac) for a 90% ECCC material
AC = exchangeable acidity (meq/100 cm3)
Target pH = the desired soil pH for the crop to be planted
Water pH = the soil pH measured in a soil-water slurry with a 1:1 soil to solution ratio.
To adjust base lime rates calculated for the Mehlich and Modified Mehlich buffers for alternative liming materials, use the following equation:

Where:
LRM = lime rate (ton/ac) based on the Mehich or Modified Mehlich buffer test for a 90% ECCC material
ECCC = effective calcium carbonate equivalent expressed as a decimal (e.g., 0.67 for agricultural-grade limestone with 67% ECCC).
SMP/Sikora Buffer
These equations were based on laboratory incubation calibration of soils by University of Kentucky researchers and are recommended for use in precision agriculture programs (Ritchie & McGrath, 2020). These equations are applicable for determining the lime recommendation using both the SMP and Sikora buffer methods.
The first step is to calculate the “lab incubation lime requirement” (LRlab; tons/ac) using the following equation:

Where:
LRlab= lime rate in ton/ac of pure CaCO3 (100% effective CCC) needed to raise the soil to the target pH in a laboratory incubation
Target pH = the desired soil pH for the crop to be planted
Water pH = the soil pH measured in a soil-water slurry with a 1:1 soil to solution ratio
Buffer pH = Sikora or SMP buffer pH
Soil mass = the weight of soil used in the measurement of water and buffer pH in grams (typically 10 g for weight-based methods or 11.8 g for a 10-cc volumetric scoop).
The LRlab rate must then be corrected to provide the appropriate LR to correct soil pH at the field scale (LRfield).
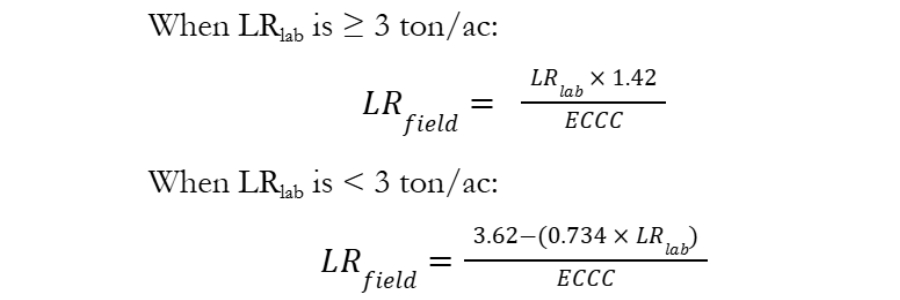
Where:
LRfield = lime rate based on Sikora or SMP buffer expressed in ton/ac of liming material
ECCC = effective calcium carbonate equivalent expressed as a decimal (e.g., 0.67 for agricultural-grade limestone with 67% ECCC).
Summary
Maintaining optimal soil pH is a cornerstone of successful crop production. While selecting a target pH and choosing the right liming materials are crucial initial steps, accurately determining the lime requirement is also important. Accurately determining the lime requirement involves understanding the interaction of active and reserve acidity in your soil, as well as considering factors like soil properties, existing pH, and the specific analytical method used by your soil testing laboratory.
Different lime requirement tests, such as various buffer pH tests (e.g., Adams-Evans, Mehlich, SMP/Sikora, and their modified, more environmentally friendly counterparts), exchangeable acidity measurements, and estimations based on soil pH and other soil properties, are used across different regions to provide the most accurate recommendations for lime rate. In Delaware, the Adams-Evans buffer pH test is commonly used, although regional labs may offer other tests. For common buffer pH tests, the base lime requirement can be calculated from specific equations or obtained from pre-established lookup tables. The selection of the appropriate lime requirement test is critical for avoiding over- or under-liming, both of which can negatively impact crop health and economic viability. Ongoing national research efforts, like the NSRP-11 project, continue to refine and improve these testing methodologies, ensuring that farmers and crop consultants have the best possible tools for effective soil pH management. By utilizing these lime requirement tests, you can create a liming program that promotes healthy plant growth, maximizes yields, and supports long-term soil sustainability.
References
Alley, M. M., & Zelazny, L. W. (1987). Soil Acidity: Soil pH and Lime Needs. In J. R. Brown (Ed.), Soil testing: Sampling, correlation, calibration, and interpretation (pp. 51-59). https://doi.org/10.2136/sssaspecpub21.c7
Hardy, D. H. (2014). Mehlich and modified Mehlich buffers for lime requirement. In F. J. Sikora & K. P. Moore (Eds.), Soil test methods from the southeastern United States (Southern Cooperative Series Bulletin No. 419, pp. 72-76). Southern Cooperative Series. http://aesl.ces.uga.edu/sera6/PUB/MethodsManualFinalSERA6.pdf
Ritchey, E., & McGrath, J. (2020). 2020-2021 Lime and Nutrient Recommendations, AGR-1. University of Kentucky Cooperative Extension Service, Lexington, KY. https://publications.ca.uky.edu/agr-1
Shober, A. L., Gartley, K. L., Miller, J. O., & Taylor, R. W. (2024). Nutrient management recommendations. University of Delaware. http://www.udel.edu/008353
Sikora, F. J. (2014). Sikora buffer for lime requirement. In F. J. Sikora & K. P. Moore (Eds.), Soil test methods from the southeastern United States (Southern Cooperative Series Bulletin No. 419, pp. 59-62). Southern Cooperative Series. http://aesl.ces.uga.edu/sera6/PUB/MethodsManualFinalSERA6.pdf
Sims, J. T. (1996). Lime Requirement. In D. L. Sparks, A. L. Page, P. A. Helmke, R. H. Loeppert, P. N. Soltanpour, M. A. Tabatabai, C. T. Johnston, & M. E. Sumner (Eds.), Methods of soil analysis (pp. 583–602). https://doi.org/10.2136/sssabookser5.3.c17
Sims, J. T., & Dennis, L. (1989). Evaluation of lime requirement methods for Delaware soils. Communications in Soil Science and Plant Analysis, 20(13-14), 1279-1296. https://doi.org/10.1080/00103628909368151
Van Lierop, W. (1990). Soil pH and Lime Requirement Determination. In R. L. Westerman (Ed.), Soil testing and plant analysis. https://doi.org/10.2136/sssabookser3.3ed.c5
About the Authors
Amy L. Shober (corresponding author), Professor and Extension Specialist, University of Delaware, Newark, DE (ashober@udel.edu)
Karen Gartley, Director, University of Delaware Soil Testing Program, Newark, DE
J. Thomas Sims, Professor (retired), Environmental Soil Management, University of Delaware, Newark, DE
About this Publication
Original publication date: 2025
Based on an original publication by J.T. Sims & K. Gartley (1996)
Peer Reviewers
Sydney Riggi, Nutrient Management Extension Agent, University of Delaware Extension, Dover, DE
Drew Harris, Agriculture Extension Agent, University of Delaware Extension, Dover, DE
UD Cooperative Extension
This institution is an equal opportunity provider.
In accordance with Federal law and U.S. Department of Agriculture policy, Cooperative Extension is prohibited from discriminating on the basis of race, color, national origin, sex, age, or disability.
