

Mastering molecule mechanics
Photo illustration by Jeffrey C. Chase January 16, 2024
UD researchers shed light on formulation, manufacturing challenges in diabetes, obesity medications
Medications such as Ozempic and Mounjaro are composed of molecules called acylated peptides that are designed to circulate in the body and regulate insulin production. This enables adults with Type 2 diabetes to take a weekly injection instead of monitoring their insulin levels every few hours. With slight modification, this class of therapeutics is also approved for weight-loss use in the treatment of obesity.
But these molecules sometimes become unstable when in contact with certain container surfaces, making formulation and manufacturing of these types of medications challenging.
University of Delaware Professor Norman Wagner and a team of researchers worked with partners at the pharmaceutical company Eli Lilly to study why this class of materials experiences this instability, a phenomenon known as ouzo formation, which can make the solution cloudy when in contact with certain surfaces designed to repel water, rendering the medication unusable.
The research team studied how the peptides behaved when in contact with surfaces such as glass, thermoplastics and synthetic polymers, to understand the fundamental mechanics behind what was happening in order to provide solutions for reducing drug product manufacturing failure.
The researchers reported their findings in a paper in the Proceedings of the National Academy of Sciences (PNAS), offering valuable insights to guide peptide synthesis, formulation, manufacturing and storage of this class of molecules. It’s work that could help across this class of medications.
In addition to Wagner, co-authors on the paper from Eli Lilly and Company include Ken Qian, director, and Kevin Seibert, vice president. Other co-authors from UD include the paper’s lead author Qi Li, a former postdoctoral researcher in UD’s chemical and biomolecular engineering department and Center for Neutron Science, and Vasudev Tangry, a former undergraduate student on the project.
Stability is key
The commonality, Wagner explained, is that drug formulations need to remain stable in solution, so the molecules don’t form large aggregates, which can be harmful if injected into the body.
“It's a fairly tricky pathway to go from a stable molecule in solution and formulation to an unstable one,” said Wagner, Unidel Robert L. Pigford Chair, chemical and biomolecular engineering at UD. “It involves the molecule adsorbing, or adhering, onto a surface like a container and creating small aggregates that become a nuclei for the growth of visible aggregates, leading to a solution that looks like the classic drink ouzo.”
Ouzo is a Greek liquor that is stable on its own but becomes cloudy when mixed with water. The cloudiness occurs because the anise contained in the liquor is not water soluble, so the droplets stay in solution, creating the drink’s murky appearance.
This might be okay for a refreshing libation, but when it happens to molecules in the pharmaceutical industry, it's problematic. It also can be costly.
“So, I might have a vessel where I am making this molecule and, in final stages, this vessel might contain something like the equivalent of over a million dollars’ worth of drug substance,” said Wagner. “If that solution forms an ouzo, you've got a real problem. You've now lost that production.”
It’s not just a production problem either. If a formulation of drug product formed an ouzo while being transported to or stored at a doctor's office, the result would be the same—it would have to be thrown out. So, on both the production side and on the storage and delivery side of the formulation, preventing this from occurring is critical.
How they approached the problem
The Wagner group’s work focused on predicting what surfaces cause problems and how rapidly this ouzo effect might occur. The research team used light and X-ray scattering along with other techniques to investigate how the molecules interacted with each other and solution. They also looked at how the molecules interacted with numerous different types of surfaces, ranging from glass to polystyrene and polytetrafluoroethylene, all common materials used in the industry.
The researchers measured the spherical shape, size, structure and the internal composition of the droplets in the solution, too. Their analysis revealed that the droplet formation was sparked by the water-repellent nature of the surfaces and depended on the rate at which the particles were stirred and mixed. The size of the particles was affected by the solution’s salt concentration, independent of the surface material. Interestingly, it seems that the particles remained in solution (rather than sinking to the bottom) due to interaction between the surface tension of the solution and the particles’ electric charge.
While explaining, Wagner gave the example of oil and water — a classic example of two types of molecules that separate when left alone. In salad dressing, two solutions are emulsified, shaken and mixed up, while products called surfactants sit at the interface between the solutions to prevent the molecules from aggregating and separating. This allows salad dressings to remain mixed over long time periods.
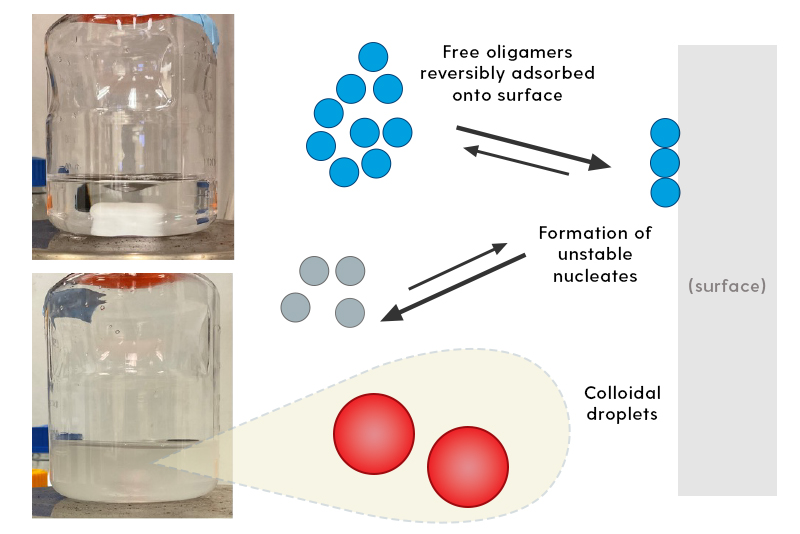
“In this case, though, we don't have any surfactants to create this, so it's curious. If it's going to separate, it should just separate like oil and water. But they don't, the molecules stay in this emulsion,” said Wagner.
The UD team applied a well-established theory from Lord Rayleigh, a noted mathematician and physicist from the University of Cambridge, that showed the droplet size and stability could be predicted and thus controlled, connecting the phenomena to many other, naturally observed phenomena. Lord Rayleigh was awarded the 1904 Nobel Prize in Physics for investigations on the densities of the most important gasses and the discovery of argon.
Wagner pointed to UD’s deep history in colloid and interface science, surfactant self-assembly, and strengths in biophysical and biomolecular systems, as advantages in exploring this type of multifaceted problem.
“There's a scientific and engineering ecosystem here at Delaware that positions us to address problems like this, because these are inherently physical chemistry problems with engineering implications in the biopharmaceutical and pharmaceutical industries,” he said. “You need all these pieces together to understand these specific molecules with very specific chemistries that are surfactant-like in some ways.”
Additional questions for future research
Knowing how an ouzo forms is one factor, understanding how long it will take for an ouzo to form is a separate question. This is because medications, like many other materials used in our everyday world, are not static. They are slowly aging.
For example, think about the way plastics may be pliable when new, but become brittle as they age. If you are talking about a pair of wireless earbuds, the material’s life expectancy may not matter much. No one expects them to be around in a hundred years, so engineering the plastic to last for five or 10 years is just fine.
But for medication, specificity matters.
Understanding how rapidly these molecules age or how long it takes for an aggregation to form under the right conditions is important. Understanding how long medications will remain shelf-stable can affect distribution and use timelines.
“Right now, we only know that this chemistry and that chemistry don't play well together, and we can predict that,” said Wagner. “Now that we put the pieces together from a chemistry perspective, we want to understand what’s going on at a molecular level that causes this and under what conditions.”
Future work by the Wagner group will employ neutron scattering techniques to look inside the droplets in detail to ask what’s going on within the molecules and their structure. Understanding what’s happening inside the droplets, on an interface in a solution could provide insight on ways to modify or change the medicine’s molecular formulation to prevent the ouzo effect from occurring despite the container it’s in.
“Right now, we can control this problem by changing the surfaces,” said Wagner. “The science question that we're asking next is whether there is something specific with this molecular structure that we could modify or change that would eliminate the problem in the molecules themselves.”
The research team also plans to examine materials related to this work that were sent over 250 miles above Earth to the International Space Station, to determine if gravity had any impact on the ouzo effect occurrence.
Contact Us
Have a UDaily story idea?
Contact us at ocm@udel.edu
Members of the press
Contact us at 302-831-NEWS or visit the Media Relations website

