

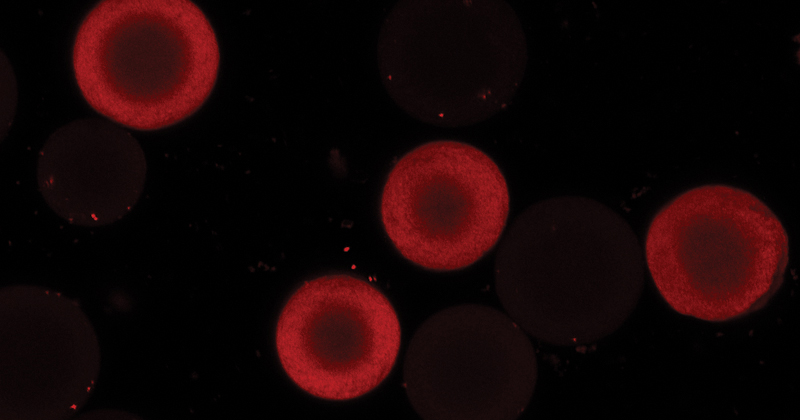
A new understanding of protein movement
Photo courtesy of Ohnmar Khanal, Vijesh Kumar and Abraham Lenhoff June 30, 2020
UD engineers uncover role of surface diffusion in protein transport, which could aid biopharmaceutical processing
Many of the most promising medicines under development are proteins, often antibodies, to help patients fight disease. These proteins must be purified as part of the manufacturing process — a task that can be tricky and result in costly waste.
Scientists have struggled to directly measure the movement of proteins, known as protein diffusion, in materials that include both solid and liquid components. They have also disagreed on how movement at the surface of the material contributes to protein movement when using ion-exchange chromatography, a laboratory and manufacturing method for separating materials based on their charge. Proteins can creep into the pores of resin beads used to perform ion-exchange chromatography and bind on the walls, based on charge.
Now, a team of engineers from the University of Delaware, with a collaborator from pharmaceutical company Amgen, has shown that surface diffusion in protein transport into ion-exchange beads depends on adsorption affinity — a measure of attraction between the two materials. By exploiting this relationship, the team developed a procedure to purify a monoclonal antibody — a type of molecule that mediates immunity — with productivity 43% higher than usual.
The team’s results were published in the Proceedings of the National Academy of Sciences in March. The paper’s authors include Ohnmar Khanal, a doctoral student in chemical engineering; Vijesh Kumar, postdoctoral fellow in chemical engineering; Fabrice Schlegel, a principal engineer at Amgen; and Abraham Lenhoff, Allan P. Colburn Professor of Chemical Engineering.
“We present a very strong case for the significance of surface diffusion, and we use multiple approaches to corroborate its importance through a simple technique that can be implemented right away,” said Khanal.
The team used chromatography, mechanistic modeling, confocal microscopy and small-angle neutron scattering. The latter was performed at the National Center for Neutron Research at the National Institute for Standards and Technology.
By understanding and exploiting protein surface diffusion in ion-exchange chromatography, researchers can build upon this work and develop methods to reduce waste during the expensive drug manufacturing process.
“Ion-exchange chromatography of proteins is an absolutely key operation in biopharmaceutical manufacturing,” said Lenhoff.
Kumar and Lenhoff are now working on a separate project funded by the National Institute for Innovation in Biopharmaceutical Manufacturing, based at the University of Delaware, to develop mathematical models of chromatography, which could enable more efficient ways of designing and developing manufacturing processes.
Researchers can also build upon this new fundamental understanding of protein diffusion and perhaps apply it to other problems. Protein diffusion on surfaces is an important phenomenon inside the body, too. Movement and fibrillation of amyloid-ß in the brain has been associated with neurogenerative diseases, for example, and protein surface diffusion can affect the performance of biosensors.
“This is an example of how fundamental research can lead to practical applications and significant improvements in those practical applications,” said Lenhoff.
And it all started with a brainstorm, where Khanal suggested more in-depth investigation of surface diffusion’s relationship to binding affinity on the charged surfaces using complementary tools.
“When we started this, we never thought we would go this far,” said Kumar. “It started as a very small idea.”
Contact Us
Have a UDaily story idea?
Contact us at ocm@udel.edu
Members of the press
Contact us at 302-831-NEWS or visit the Media Relations website

