

Lens Crafting
May 17, 2018
NIH director Francis Collins highlights work by UD researchers
Editor's Note: Francis Collins, director of the National Institutes of Health, wrote a blog post on May 17, about eye research done by a team from the University of Delaware. The blog post is reprinted here with permission of NIH. The image used in this story and Dr. Collins' blog post is from the lab of UD biologist Salil Lachke.
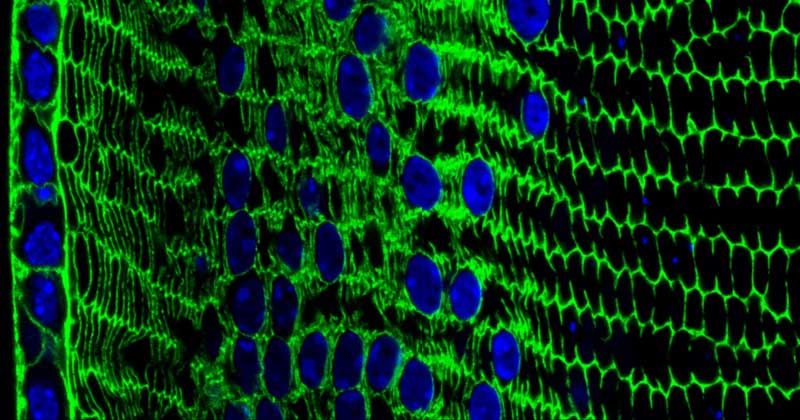
Live long enough, and there’s a good chance that you will develop a cataract, a clouding of the eye’s lens that impairs vision. Currently, U.S. eye surgeons perform about three million operations a year to swap out those clouded lenses with clear, artificial ones [1]. But wouldn’t it be great if we could develop non-surgical ways of preventing, slowing, or even reversing the growth of cataracts? This image, from the lab of NIH-grantee Salil Lachke at the University of Delaware, Newark, is part of an effort to do just that.
Here you can see the process of lens development at work in a tissue cross-section from an adult mouse. In mice, as in people, a single layer of stem-like epithelial cells (far left, blue/green) gives rise to specialized lens cells (middle, blue/green) throughout life. The new cells initially resemble their progenitor cells, displaying nuclei (blue) and the cytoskeletal protein actin (green). But soon these cells will produce vast amounts of water-soluble proteins, called crystallins, to enhance their transparency, while gradually degrading their nuclei to eliminate light-scattering bulk. What remains are fully differentiated, enucleated, non-replicating lens fiber cells (right, green), which refract light onto the retina at the back of the eye.
While water continually cycles in and out of the lens fiber cells, their crystallin proteins remain the same over time, gradually denaturing from exposure to ultraviolet rays in sunlight and other environmental factors. As they denature, the crystallins clump together in the lens, forming cataracts that block light transmission.
Currently, there’s no way to stop or reverse the process of cataract formation. But recent basic research suggests it may be possible to do so if we can identify the precise biological cause of crystallin aggregation and develop drugs aimed at that biological target [2, 3].
That’s why Lachke and other researchers are hard at work defining the genetic circuitry activated during lens development.[4] Already, Lachke’s group has assembled genetic profiles of lens tissue at different timepoints during mouse development, as well as created a free bioinformatic navigational tool for the datasets called iSyTE (integrated Systems Tool for Eye gene discovery). [5] Researchers can use iSyTE to analyze and prioritize genes that could be important for keeping the lens as transparent as possible in humans over the course of our lifetimes. [6 – 9].
Also, Lachke’s work has generated lots of wonderfully informative images of the eye lens. This one, snapped with a confocal microscope by grad student Salma Muhammad Al Saai, was among those highlighted in the University of Delaware’s 2017 Art in Science exhibit. With its gorgeous greens and blues, this micrograph helps to brings into focus a health challenge that many of us will face as we grow older.
References:
[1] Ambulatory Surgery Data From Hospitals and Ambulatory Surgery Centers: United States, 2010, Hall MJ, Schwartzman A, Zhang J, Liu X. National Health Statistics Reports: 102. 2017 Feb 28. National Centers for Disease Control and Prevention.
[2] Lanosterol reverses protein aggregation in cataracts. Zhao L, Chen XJ, Zhu J, Xi YB et al. Nature. 2015 Jul 30;523(7562):607-611.
[3] Pharmacological chaperone for α-crystallin partially restores transparency in cataract models. Makley LN, McMenimen KA, DeVree BT, Goldman JW, McGlasson BN, Rajagopal P, Dunyak BM, McQuade TJ, Thompson AD, Sunahara R, Klevit RE, Andley UP, Gestwicki JE. Science. 2015 Nov 6;350(6261):674-677.
[4] Systems biology of lens development: A paradigm for disease gene discovery in the eye. Anand D, Lachke SA. Experimental Eye Research. 2017 Mar;156:22-33.
[5] iSyTE 2.0: a database for expression-based gene discovery in the eye. Kakrana A, Yang A, Anand D, Djordjevic D, Ramachandruni D, Singh A, Huang H, Ho JWK, Lachke SA. Nucleic Acids Research. 2018 Jan 4;46(D1):D875-885.
[6] Mutations in the RNA granule component TDRD7 cause cataract and glaucoma. Lachke SA, Alkuraya FS, Kneeland SC, Ohn T, Aboukhalil A, Howell GR, Saadi I, Cavallesco R, Yue Y, Tsai AC, Nair KS, Cosma MI, Smith RS, Hodges E, Alfadhli SM, Al-Hajeri A, Shamseldin HE, Behbehani A, Hannon GJ, Bulyk ML, Drack AV, Anderson PJ, John SW, Maas RL. Science. 2011 Mar 25;331(6024):1571-1576.
[7] RNA‐binding proteins in eye development and disease: implication of conserved RNA granule components. Dash S, Siddam AD, Barnum CE, Janga SC, Lachke SA. Wiley Interdisciplinary Reviews: RNA 2016 Jul;7(4):527-557.
[8] Compound mouse mutants of bZIP transcription factors Mafg and Mafk reveal a regulatory network of non-crystallin genes associated with cataract. Agrawal SA, Anand D, Siddam AD, Kakrana A, Dash S, Scheiblin DA, Dang CA, Terrell AM, Waters SM, Singh A, Motohashi H, Yamamoto M, Lachke SA. Human Genetics. 2015 Jul;134(7):717-735.
[9] The RNA-binding protein Celf1 post-transcriptionally regulates p27Kip1 and Dnase2b to control fiber cell nuclear degradation in lens development. Siddam AD, Gautier-Courteille C, Perez-Campos L, Anand D, Kakrana A, Dang CA, Legagneux V, Mereau A, Viet J, Gross JM, Paillard L, Lachke SA. PLOS Genetics. 2018 Mar 22;14(3):e1007278.
Links:
Cataract (National Eye Institute, NIH)
Insight into the Eye Lens (University of Delaware, Newark)
Salil A. Lachke (University of Delaware)
iSyTE (University of Delaware)
University of Delaware 2017 Art in Science
NIH Support: National Eye Institute
Contact Us
Have a UDaily story idea?
Contact us at ocm@udel.edu
Members of the press
Contact us at 302-831-NEWS or visit the Media Relations website

