

Innovation at UD: Jason Gleghorn: youtube.com/watch?v=3fk7c0uFdWQ
Innovation Ambassador: Jason Gleghorn
Video by Ally Quinn and Sam Kmiec | Photo illustration by Jeffrey C. Chase | Photos by Kathy F. Atkinson October 01, 2025
Taking discoveries to the real world for the benefit of human health
Editor’s note: The University of Delaware is diligently working to enhance infrastructure and support available to campus innovators. As part of this effort, the U.S. National Science Foundation's Accelerating Research Translation program (NSF ART program) at UD is investing in capacity-building resources to boost the translation of UD research discoveries into novel technologies of benefit to Delawareans and the nation. UD is an inaugural member of the NSF ART program.
It takes about a decade and a lot of money to bring a new drug to market—between $1 billion to $2 billion, in fact.
University of Delaware inventor Jason Gleghorn wants to change that.
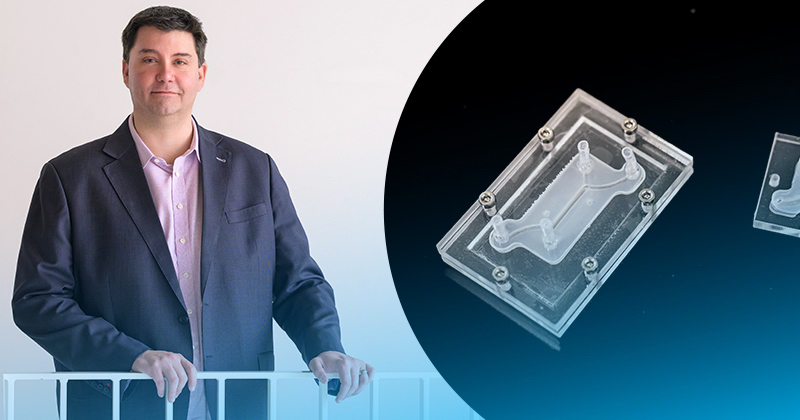
At UD, Gleghorn is developing leading-edge microfluidic tissue models. The devices are about the size of two postage stamps, and they offer a faster, less-expensive way to study disease and to develop pharmaceutical targets.
These aren’t tools he wants to keep just for himself. No, Gleghorn wants to put the patented technology he’s developing in the hands of other experts, to advance clinical solutions in women’s health, maternal-fetal health and pre-term birth. His work also has the potential to improve understanding of drug transport in the female reproductive tract, placenta, lung and lymph nodes.
Gleghorn, an associate professor of biomedical engineering, was named to the first cohort of Innovation Ambassadors at UD, as part of the University’s effort to foster and support an innovation culture on campus. Below, he shares some of what he’s learned about translating research to society.
Q: What is the problem that you are trying to address?
Gleghorn: A lot of disease has to do with disorganization in the body’s normal tissue structure. My lab makes microfluidic tissue models, called organ-on-a-chip models, that have super-tiny channels about the thickness of a human hair, where we can introduce very small amounts of liquid, including cells, to represent an organ in the human body. This can help us study and understand the mechanism of how things work in the body (the biology) or help us do things like drug screening to test therapeutic compounds for treating disease.
And while these little microfluidic devices can do promising things, the infrastructure required to make the system work often restricts their use to high-end labs. We want to democratize the techniques and technology so that nonexperts can use it. To achieve this, we changed the way we make these devices, so that they are compatible with standard manufacturing, which means we can scale them and create them much easier.
Q: What is the benefit of this work to the public?
Gleghorn: One of the problems with drug screening, in general, is that animal model studies don’t always represent human biology. So, when we’re using animal models to test new drugs — which have been the best tool we have available — the results are not always apples to apples. Fundamentally, our microfluidic devices can model what happens in humans … we can plug in the relevant human components to understand how the mechanism is working and then ask questions about what drives those processes and identify targets for therapies to prevent the dysfunction.
Q: What is innovative about this device?
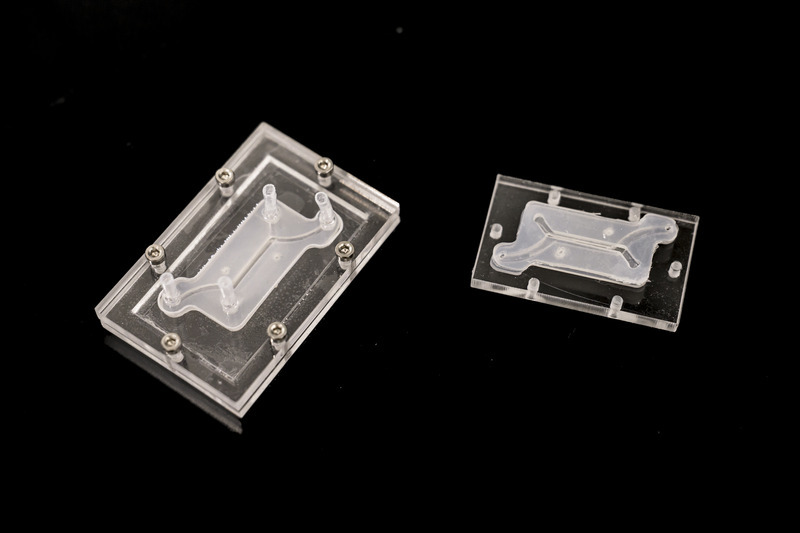
Gleghorn: The innovation part is this modularity — no one makes these devices this way. The science happens on the tiny tissue model insert, which is sandwiched between two pieces of clear acrylic. This allows us to watch what’s happening on the tissue model insert in real time. Meanwhile, the outer shell’s clamshell design provides flexibility: if we’re studying lung tissue and we want to study the female reproductive tract, all we do is unscrew the outer shell and insert the proper tissue model that mimics the female reproductive tract and we’re off. We’ve done a lot of the engineering to make it very simple to operate and use, and adaptable to common lab tools that everyone has, to eliminate the need for financial investment in things like specialized clean rooms, incubators and pumps, etc., so the technology can be useful in regular labs or easily deployable to far-flung locations or countries. With a laser cutter and $500 worth of equipment, you could conceivably mass manufacture these things for maternal medicine in Africa, for example.
Democratizing the technology so it is compatible and useful for even an inexperienced user aligns with the mission of my lab, which focuses on scaling the science and the innovation faster, instead of only a few specialized labs being a bottleneck to uncovering new mechanisms of disease and the development of therapies. We patented this modularity, the way to build these tiny microfluidic devices and the simplicity of how it's used as a tool set, through UD’s Office of Economic Innovation and Partnerships (OEIP).
Q: How have you translated this work so far?
Gleghorn: To date, we've taken this microfluidic system to nine different research labs across seven countries and four continents — including the United States, the United Kingdom, Australia, France, Belgium and South Africa. These labs are using our technology to study problems in women’s health and collecting data with it. We’re developing boot camps where researchers can come for two or three days to the University of Delaware, where we teach them how to use this device and they take some back with them. From a basic science perspective, there is high enthusiasm for the power of what it can tell you and its ease of use. As engineers, we think it's pretty cool that many other people are using our innovations for new discoveries.
Q: What support and guidance have you received from the UD innovation ecosystem?
Gleghorn: To do any of this work, you need partners that have various expertise and backgrounds. UD’s Office of Economic Innovation and Partnerships has built a strong team of professionals with expertise in different areas, such as how do you license or take something to patent, how do you make connections with the business community? OEIP is home to Delaware’s Small Business Development Center, which can help you think about business visibility in terms of startups. Horn Entrepreneurship has built out impressive programs for teaching students and faculty to think entrepreneurially and build mentor networks, while programs like the Institute for Engineering Driven Health and the NSF Accelerating Research Translation at UD provide gap funding to be able to do product development and to take the work from basic prototype to something that is more marketable. More broadly in Delaware is the Small Business Administration, the Delaware Innovation Space and regional grant programs and small accelerators to help Delaware innovators.

Q: How have students in your lab benefited from engaging in innovation?
Gleghorn: Undergraduate students in my lab have made hundreds of these devices at scale. We basically built a little manufacturing facility, so we have ways to sterilize them, track batches, etc. We call it “the foundry.” In other work, graduate students are engineering different components or working on specific system designs for various studies. The students see collaborators use these devices to discover new science and new discoveries. That's very rewarding as an engineer. Additionally, my lab focuses on building solutions that are useful in the clinic and commercially viable. As a result, we've had two grad students spin out companies related to the work we've been doing in the lab.
Q: How has research translation positively impacted your work?
Gleghorn: I started down this road maybe five years ago, seriously trying to think about how to translate our research findings. Being an entrepreneur, translating technology — it's a very different way to think about your work. And so that framework has really permeated most of the research that I do now and changed the way I think about problems. It has opened new opportunities for collaboration and for alternate sources of funding with companies. This has value in terms of taking the research that you're doing fundamentally and creating a measurable impact in the community, but it also diversifies your funding streams to work on important problems. And different viewpoints help you look at the work you do in new ways, challenging you to define the value proposition, the impact of your work.
Contact Us
Have a UDaily story idea?
Contact us at ocm@udel.edu
Members of the press
Contact us at mediarelations@udel.edu or visit the Media Relations website

