Getting to the guts of disease
OUR FACULTY | The gluten-free movement. Beyonce’s vegan meal plan. “The Whole 30.” Pretty much any diet promising good health and real results.
If there’s one common denominator, according to Prasad Dhurjati, it’s this: gut bacteria.
“To the extent you can control your gut bacteria, you can control your health,” says the professor of chemical and biomolecular engineering.
You read that right—engineering.
Dhurjati is an elected Fellow in the American Institute of Medical and Biological Engineering, but he’s not a health professional.
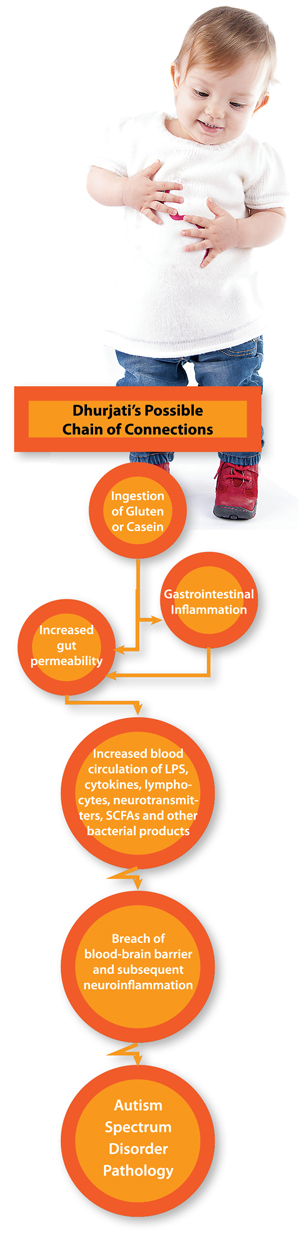
And yet when he began to study autism a few years ago—after speaking to a colleague whose son exhibited stomach symptoms and autism—Dhurjati discovered a novel way to look at the disorder. In the first paper he’s ever written on the subject, he and former student Colin Heberling, EG10, described a possible chain of connections that could be delivering toxic messages from the gut to the brain in people on the autism spectrum.
The work, published in the peer-reviewed journal Medical Hypothesis in 2013 and hailed on Delaware’s NPR station by a Harvard neurology professor as “a vital stepping stone in the long path from research to treatments” is, in the words of Dhurjati, “actually quite basic.”
The autism elephant
In the unfunded study he took on out of sheer curiosity, Dhurjati examined possible primary causes of autism, including abnormal intestinal bacteria, the food and products of such gut microbes, bacterial toxins, dietary allergens and more.
His goal: To look at publications that implicated different factors involved in autism and build a map of how they connected.
“I handed the 40 or so papers to my students and said, ‘For every paper or concept, draw a box. And after that, connect the boxes.’”
This is the fundamental tenet of systems engineering: The ability to understand how the components of any system, anywhere—space shuttle, chemical plant, human body—work together.
Dhurjati describes his work using the ancient Indian story of the blind men and the elephant. The man who grabbed the tail “saw” a rope. The one who felt the tusk, a pipe. The third who touched the trunk, a tree branch. And so on. Dhurjati’s work attempts to see the full “autism elephant.” His second paper on the topic replaced lines with equations and resulted in the first mathematical model of autism. The third added rules and built the first artificial intelligence model of autism: “If bad stuff is getting out of the digestive track and reaching the brain, then how is intestinal and blood-brain permeability a function of the bad stuff?”
His research essentially maps the brain-gut connection in people with autism to assess how all the parts work together.
At the core to better understanding this disorder and “virtually every disease out there,” he says, is the digestive tract and the 100 trillion-plus microbes that live there.
But isn’t that just one piece of the elephant?
Food for thought
Dhurjati is quick to say no. For every human cell we have in our bodies—our central DNA make-up—there are 10-times as many microbial guest cells. For every gene we inherit from our parents, we have 100-times more genes in the gut cells.
“Gut microbes have 100-times more genes than what your parents give you,” he explains. “It’s almost as if microbes are using you to grow, and not the other way around.”
The trillions of microbes live everywhere in the human body. They vary. No two bodies—and consequently, no two guts—are the same. This diversity leads to personalized “precision medicine” therapies. Dhurjati’s focus now is on taking a systems engineering approach, much like he did with autism, to better understand the body’s ever-changing microbial balance.
The digestive system, he says, is our most critical bioreactor, absorbing and converting food into materials that will renew the body. And, “We can change our microbial composition through food.”
Dhurjati’s next area of scholarship is using engineering to learn how foods continually influence changes in the gut microbial population, how the gut population impacts disease and, ultimately, how we alter the gut population to move from a state of disease to health.
In many ways, we have ancient history to guide us, he says, quoting Hippocrates, whose oath doctors across the world swear to uphold: “Let food be thy medicine and medicine thy food.”
Article by Artika Rangan Casini, AS05




