Remineralization
UD-led study suggests new pathway for phosphorous cycling in Chesapeake Bay
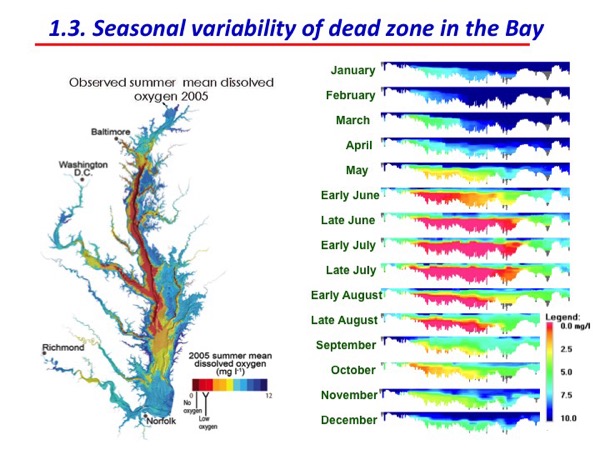
12:51 p.m., Feb. 19, 2015--In the summer months, phosphorous cycling leads the center of the Chesapeake Bay to suffer from bottom water hypoxia — low levels of oxygen — which makes it hard for oxygen dependent organisms to survive. Conversely, this cycling also causes surface water eutrophication, which leads to phytoplankton blooms.
In a new paper published in the journal Environmental Science and Technology, researchers from the University of Delaware and other institutions have identified for the first time organic matter remineralization as the predominant pathway for the phosphorous cycling that occurs in the Chesapeake.
Research Stories
Chronic wounds
Prof. Heck's legacy
The research, led by Sunendra Joshi, a doctoral student in the College of Agriculture and Natural Resources, includes UD’s Deb Jaisi, assistant professor in the Department of Plant and Soil Sciences, and Donald Sparks, the S. Hallock du Pont Chair in the Department of Plant and Soil Sciences, Francis Alison Professor, director of the Delaware Environmental Institute (DENIN) and a leader of the University’s Environmental Soil Chemistry Group.
The UD scientists collaborated with David Burdige, professor and Eminent Scholar in the Department of Ocean, Earth and Atmospheric Sciences at Old Dominion University; Ravi Kukkadapu, a senior research scientist at the Environmental Molecular Science Laboratory (EMSL) at the Pacific Northwest National Laboratory; and Mark Bowden, a scientist at EMSL.
Remineralization vs. remobilization
Remineralization is a cycling process that starts with the breaking down of organic matter, in this case phytoplankton blooms that take up phosphorous and live in top level waters that have undergone eutrophication.
When those phytoplankton die, they settle to the bottom of the body of water, break down and release phosphorous, causing hypoxia.
While some believe the dead zone in the center of the Chesapeake during the midsummer months can be attributed to nutrient remobilization — phosphorous entering from terrestrial or atmospheric sources, settling to the sediment and then again mobilizing to bottom water — this research suggests that the problem lies in organic matter remineralization, as it forms the predominant pathway for phosphorous cycling in this section of the bay.
“This remineralization process is more of a natural process of cycling and leans more toward a self-sustaining process than having direct phosphorous coming into the bay from the land,” said Jaisi. “That [remobilization] pathway is almost not there. It is very insignificant because the bulk of land driven phosphorus is buried in the sediment and is inactive. People used to think that that pathway is a significant pathway compared to remineralization, which is not the case. It’s the other way around, and that has a direct impact on how to control the nutrient issue in the bay.”
Seasonal event
The late spring, early summer months are the perfect time for the eutrophication process to begin as they follow the spring when the bay is fed by heavy water flows from the rivers, which bring a substantial amount of nutrients into the Chesapeake. In the early summer, the temperatures are right for organisms like phytoplankton to grow.
Burdige explained that, like every plant, phytoplankton requires several basic things in order to grow, including light, the correct temperature and nutrients.
“It’s similar to how you put fertilizer on your garden to make tomatoes grow, or your grass grow. Plankton need nitrogen and phosphorous,” said Burdige, who added that a spring phytoplankton bloom “is not dissimilar to what happens to your lawn. Your lawn dies in the winter and then, come around March or April, it starts to be green again.”
The phytoplankton contribute to phosphorous cycling because in summer months when they die, they break down and their remains sink to the bottom water and to the sediment column. The nutrients that the phytoplankton took up to form themselves in the first place regenerate and are returned to the bottom water to refuel the dead zone and may diffuse up to the surface water to feed a new generation of phytoplankton.
“So it is a recycling process, a process that reuses the same nutrient multiple times,” said Joshi.
Jaisi added that it is “an efficient system for microorganisms but a great pain for nutrient management.”
Sediment cores
In order to study the processes, the researchers extracted sediment cores from three sites in the Chesapeake — though their study only concentrated on the mid-bay portion — freeze dried them and analyzed the different phosphorous pools, taking the isotopic composition for those different pools and looking at what the isotope means and how the specific range of isotopic composition evolve.
According to the paper, this is the first natural environment where the preservation of the isotopic signature immediately after the remineralization of organic matter has been documented in the world.
“This can only happen under intense remineralization where the released phosphorus is too high to precipitate as a mineral instantly. The beauty of the isotope tool is that once the phosphate mineral precipitates, the isotopic composition is locked in and is now available for us to see what reactions and processes happened in the past,” said Jaisi.
The researchers looked at authigenic phosphorous pools — phosphorous that precipitates as a mineral — and iron oxide-bound phosphorous pools, which largely include phosphorus entering the bay from terrestrial sources and end up in the sediment.
The research showed that the iron oxide dissolution seems to be a minor source of the phosphorous in the area of the bay they studied. This means that a bulk of land driven phosphorus remains in sediment and is not active anymore.
Kukkadapu added that iron oxide bound phosphorus is not reactive as it is commonly anticipated, saying, “The small-particle iron oxides coated with organic matter and phosphorous in the uppermost layer of the sediment are surprisingly stable toward changes under anoxic conditions.”
In addition to looking at current sediments, they were also given historic sediment samples from past years in the Chesapeake thanks to Burdige, who has spent years studying the bay.
“Deb contacted me because he was looking for samples. We’ve done a lot of work in the bay over the years so we have a lot of frozen mud,” said Burdige, who pointed out that these historic sediments were studied to shine a light on legacy nutrients, those that enter the bay because of runoff but remain buried in sediment.
“If, for example, we figured out a way tomorrow to stop all inputs of excess nitrogen and phosphorous from human sources, there would still probably be a declining problem with excess nutrients in the bay because you’ve got this legacy reservoir that’s sitting on the bay floor,” said Burdige. “It’s sort of providing a new source of nutrients. It’s new today, so to speak, but it originally entered the bay 10 years ago, 20 years ago, 50 years ago. So it’s like rooting through your pantry to find a can of soup that you stashed away and forgot about.”
Analytical tools
By using a number of advanced state of the art analytical tools involving field sampling and laboratory work, and examining sediments all the way to the sub-atomic level, using Jaisi’s Environmental Biogeochemistry Laboratory to measure the phosphate oxygen isotopes, Sparks said that “one of the strengths of this work is that we’ve taken multiple approaches and really used some cutting edge techniques to try to get very precise information on the major source and what the cycling is due to.”
Sparks said that his group brought certain spectroscopic tools, collaborating with Bowden and Kukkadapu, who did the Mössbauer spectroscopy portion of the research at EMSL, and then “Deb had the isotope biogeochemistry expertise so it was a nice partnership.”
Burdige said that in a lot of ways, the result of the research is a testament to Jaisi and Joshi’s hard work “in making these difficult and challenging isotope measurements. From that perspective, they should be commended for their persistence and perseverance.”
The Soybean Boards of Maryland, Delaware and Pennsylvania provided major funding for this research, while the United States Department of Agriculture (USDA) and Delaware EPSCoR also helped fund the research.
Article by Adam Thomas
Photos by Kathy F. Atkinson and courtesy of Deb Jaisi
Images courtesy of Deb Jaisi