Solar fuel success
UD-developed solar reactor can produce solar hydrogen, but how much?
9:59 a.m., Feb. 4, 2013--Last spring University of Delaware doctoral candidate Erik Koepf and research associate Michael Giuliano spent two months in Switzerland testing a novel solar reactor Koepf developed to produce hydrogen from sunlight.
Eight weeks of sophisticated testing at temperatures up to 1,200 degrees Celsius revealed that the reactor’s mechanical, electrical and thermal systems worked just as Koepf had predicted.
Research Stories
Chronic wounds
Prof. Heck's legacy
He was even able to collect small amounts of the stored solar energy in a vial, despite operating below critical reaction temperatures in order to validate the system’s components in a high temperature environment.
Next month, Koepf heads back to the Swiss Federal Institute of Technology in Zurich to operate the reactor at full power for the first time.
“Our objective is to produce as much solar fuel as possible,” he said.
Fuels produced directly from sunlight have the potential to address concerns over energy security, declining conventional fuel production and long-term energy sustainability and environmental responsibility. It is a clean and benign energy harvesting cycle that creates no emission or by products — just hydrogen from sunlight and water.
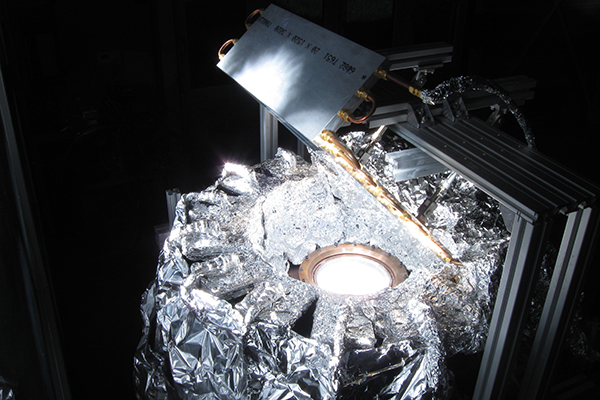
Koepf’s reactor is designed to accomplish the first step in a two-step water-splitting process to generate hydrogen renewably from sunlight. The reactor, which is closed to the atmosphere, uses gravity to feed zinc oxide powder (the reactant) into the system through hoppers that dispense the powder onto a ceramic surface. There it undergoes a thermochemical reaction upon exposure to highly concentrated sunlight within the reaction cavity, producing solar fuel.
Novel mirror assembly
In Phase I experiments, Koepf used an existing mirror assembly in place at the Swiss Federal Institute of Technology to direct the concentrated sunlight and conduct his tests. But this time, in order to achieve the extremely high temperature range needed to produce solar fuel — 1,750-1,950 degrees Celsius — he will need a mirror nearly four times the size of the existing one.
Since no such mirror exists, Koepf and Giuliano designed and built their own.
The mirror is a perfectly flat, water-cooled aluminum plate with a 98 percent reflective foil surface. About one-inch thick, the mirror measures 45 by 45 inches square and contains 13 holes bored through the one-inch thick plate along one of the mirror’s axes, enabling water to continuously pass through the channels and cool the mirror’s surface when in use.
To allow for precise adjustments in real time, the mirror is suspended above the reactor by threaded rods connected to a motor and control assembly, and controlled using a joystick. Placed on a 45 degree angle, Koepf explained, it will enable a perfectly reflected, concentrated light cone that is free of distortion to enter the reactor.
“We are concentrating an area of light that is about a 10-foot by 10-foot area into a 6 centimeter diameter circle. Under these extremely high temperatures, even being off a few millimeters, for a few seconds could significantly damage the reactor,” Koepf said.
The mirror assembly and reactor, which weigh nearly 3,000 pounds, have already been packed and shipped to Switzerland. Koepf will follow in a few short weeks. While he said it would be nice to come back with “buckets of zinc infused with solar energy” instead of just a few vials, Koepf contends that the real work goes deeper.
“We want to analyze the reactor under different conditions to determine how our production rates vary under different levels of zinc oxide dispersal, temperatures and flow patterns,” he said of optimizing the system’s solar fuel production.
Koepf’s UD advisers are Ajay Prasad, professor of mechanical engineering and director of UD’s Center for Fuel Cell Research, and Suresh Advani, department chair and George W. Laird Professor of Mechanical Engineering.
“Erik’s project is extremely ambitious and multi-disciplinary in nature. It involves heat transfer and fluid dynamics in a very high temperature, chemically-reacting environment. He has had to master the use of high temperature ceramic materials and design his reactor to withstand enormous thermal stresses. His progress to date is a testament to his strong design and analysis skills and I am confident that his second campaign in Zurich will bring exciting results,” said Prasad.
According to Advani, Koepf’s project also illustrates the value of developing partnerships, both locally and globally. His work has led to international collaboration with Aldo Steinfeld, a worldwide expert in the field and head of the Solar Process Laboratory and the Paul Scherrer Institute.
Koepf’s reactor concept and design details, along with Phase I experimental findings, can be found in the Sept. 19, 2012 issue of the International Journal of Hydrogen Energy.
Article by Karen B. Roberts
Photos by Kathy F. Atkinson and courtesy of Erik Koepf