COVID-19 RESOURCES
INFO FOR UD & EXTENSION STAFF
What are your thoughts and feelings about the COVID-19 vaccine?
Take a few moments and watch the following video featuring two highly respected members of our UD community: Dr. Joan Coker, Physician and University Trustee, and Dr. Tim Dowling, Physician and Director, Student Health Services, where they discuss their views on the vaccines and where to get trusted information, Building Community Through Vaccine Confidence.
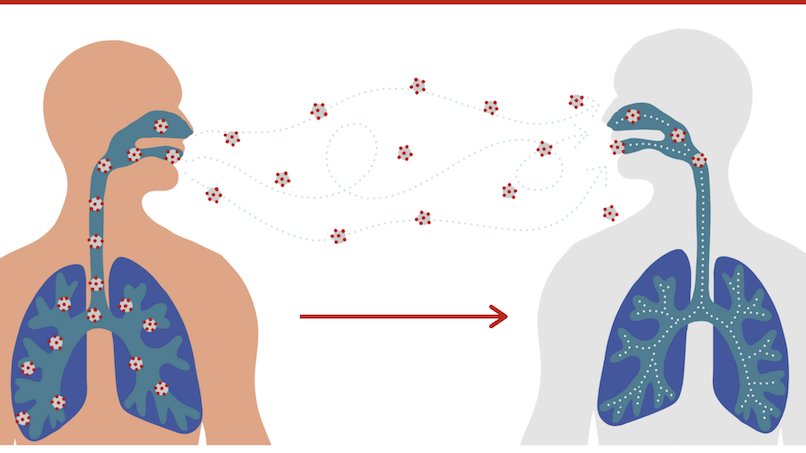
Getting the vaccine is one of the many steps you can take to protect yourself and others from COVID-19. Protection from COVID-19 is critically important because for some people, COVID-19 can cause severe illness or death.
Vaccines work with your immune system so your body will be ready to fight off the virus if you are exposed. After you are fully vaccinated against COVID-19, you may be able to start doing some things that you stopped doing because of the pandemic. You should keep taking precautions in public places or when you are with unvaccinated people from more than one household.
Getting vaccinated and following CDC’s recommendations to protect yourself and others will offer the best protection from COVID-19. For additional information, visit the official CDC vaccine webpage.
The State of Delaware is now
vaccinating persons 5+
Where to get vaccinated in DE
Where to get vaccinated in Delaware: youtube.com/watch?v=HCoaT_2wPWc
Make a vaccination appointment
How to make a vaccination appointment in Delaware: youtube.com/watch?v=s3c3AyFwJpE
Vaccine FAQs
Schools or employers may and are allowed to require vaccinations for attendance or employment, and requirements vary by state and employer
No vaccine is 100% effective at preventing infection, and highly contagious variants have led to breakthrough infections among vaccinated people, but the vaccines continue to be highly effective in reducing risk of severe disease, hospitalization, and death, and can provide sustained protection when you receive a booster dose
All COVID-19 vaccines have been rigorously tested and reviewed. The vaccine’s clinical trials three-phase process was detailed and thorough, and no shortcuts were taken.
Comparing available vaccines
*Note: These vaccines received full FDA approval
Moderna* |
Pfizer* |
Johnson & Johnson |
Novovax |
|
Type of vaccine |
mRNA |
mRNA |
viral vector |
Protein Subunit |
Number of shots |
2 shots, |
2 shots, |
1 |
2 shots, 3-8 weeks apart |
How given |
shot in the muscle |
shot in the muscle |
shot in the muscle |
shot in the muscle |
Common side effects |
|
|
|
|
How well it works |
|
|
|
|
How long until you're protected |
|
|
|
|
How the vaccines work
The COVID-19 vaccines allow us to develop immunity to the virus that causes COVID-19 without us having to get the illness. Different vaccines work in different ways, however like all vaccines, the body is left with a supply of “memory cells” that will remember how to fight the infection in the future.
It typically takes a few weeks after vaccination for the body to produce these “memory cells”. Therefore, it is possible to be infected with the virus that causes COVID-19 just before or after vaccination and then get sick because the vaccination did not have enough time to provide protection
After vaccination, the process of building immunity can cause symptoms, such as fever. This is completely normal and is a sign that the body is building immunity to the virus.
It is important to note that no vaccine is 100% effective at preventing infection, and highly contagious variants have led to breakthrough infections among vaccinated people, but the vaccines continue to be highly effective in reducing risk of severe disease, hospitalization, and death, and can provide sustained protection when you receive a booster dose.
mRNA Vaccines
mRNA vaccines contain material from the virus that causes COVID-19 that gives our cells instructions for how to make a harmless protein that is unique to the virus. After our cells make copies of this harmless protein, they destroy the genetic material from the vaccine. Our bodies recognize that this protein is not supposed to be there, and build “memory cells” that will remember how to fight the virus that causes COVID-19 if we are infected in the future.
Vector Vaccines
Vector vaccines contain a modified version of a different virus than the one that causes COVID-19. Inside the shell of the modified virus, is material from the virus that causes COVID-19. This is called “viral vector”. Once the viral vector is inside of our cells, the cells receive instructions to make a protein that is unique to the virus that causes COVID-19. Our cells then make copies of that protein. This prompts our bodies to make “memory cells” that will remember how to fight that virus if we are infected in the future.
Safety
All COVID-19 vaccines have been rigorously tested and reviewed. The vaccine’s clinical trials three-phase process was detailed and thorough, and no shortcuts were taken.
- The vaccine will protect you from becoming infected with the virus, and from getting severely ill if you are diagnosed with COVID-19.
Even though the vaccine was developed quickly, no corners were cut.
The vaccine was strictly monitored by the FDA, and more people than is standard volunteered for trials to speed up the testing process.
The CDC still monitors the safety of the COVID-19 vaccine through the use of technology.
- You can get the vaccine with confidence!
In the arm where you got the shot:
Pain
Redness
Swelling
Throughout the rest of your body:
Tiredness
Headache
Muscle pain
Chills
Fever
Nausea
These side effects happen within a day or two of getting the vaccine. They are normal signs that your body is building protection and should go away within a few days.
In clinical trials, reactogenicity symptoms (side effects that happen within 7 days of getting vaccinated) were common but were mostly mild. Some people had side effects that affected their ability to do daily activities.
Side effects (such as fever, chills, tiredness, and headache) throughout the body were more common after the second dose of the vaccine.
The Vaccine does not affect fertility or DNA. It does not make you magnetic.
There isn’t any evidence that any vaccines, including COVID-19 vaccines, cause female or male fertility problems.
COVID-19 vaccines will not make you magnetic because they do not contain ingredients that can produce an electromagnetic field.
The materials in COVID-19 vaccines never enter the nucleus of the cell, which is where DNA is located. Therefore, they do not interact with your DNA or change it in any way.
- A booster is recommended for people ages 5 years and older once they have completed their primary series doses
- Everyone ages 5 years and older is recommended to receive 1 age-appropriate bivalent mRNA booster dose two months after completion of any FDA-approved or FDA-authorized monovalent primary series or last monovalent booster dose.
- People cannot get a bivalent booster without first completing at least a primary series
- How do I know if I am up to date?
- If you have completed a COVID-19 vaccine primary series AND have received the most recent booster dose recommended for you by CDC, then you are considered up to date.
Getting COVID-19 during pregnancy is serious. It can cause you to get very sick and increase the risk of preterm birth (before 37 weeks of pregnancy).
CDC recommends COVID-19 vaccination for all people who are pregnant, breastfeeding, or trying to get pregnant now or in the future
Antibodies made after a pregnant person received an mRNA COVID-19 vaccine have been found in umbilical cord blood, which means that COVID-19 vaccination during pregnancy might help protect babies against COVID-19
There is currently no evidence that shows that any vaccines including COVID-19 vaccines, cause fertility problems in women or men.
The CDC does not know for sure if mothers with COVID-19 can spread the virus to babies in breast milk, but based on what they do know, they claim it is unlikely.
Overcoming the fear
Seek professional help. A professional therapist has knowledge and experience in helping people with phobias, such as fear of needles. A therapist can help explore the roots of your fear and offer coping skills and techniques, such as cognitive behavior therapy or exposure therapy.
Ask your doctor about medication to help manage anxiety. Some patients benefit from a topical anesthetic to numb their arm before the shot.
Look at positive posts and photos of people holding their COVID-19 vaccination cards. This may help you associate the vaccine with positive feelings.
Practice deep breathing exercises (this can help you cope during your vaccine appointment, too).
Focus on the benefits of the vaccine. The COVID-19 vaccine is a safe and effective tool to protect yourself and your family against a virus that has wreaked havoc in our lives for more than a year.
After making your appointment:
Bring someone trusted with you for support. Some vaccination centers may not allow it, but you can ask ahead of time for a special exception.
Tell the nurse about your fears before getting the shot. The nurse may be able to explain the steps, count down, etc. to give you a sense of control.
If you feel faint, tense your muscles or make a fist. You may even ask the nurse if you can get the shot lying down or stay for a few minutes after it, to prevent fainting.
Don’t look. There’s no need to look at the needle, it will only increase your anxiety.
Distract yourself. Even though it will be over in seconds, distraction can help. Listen to a song or play a video on your phone, practice deep breathing, wiggle your toes, or tune into your senses.
Even after receiving the first and second dose of the vaccine, it is important to continue to protect yourself and others. While medical experts feel very confident that the COVID-19 vaccine can protect you from the virus and help get us all back to normal, it will take a large number of people getting the vaccine before our community can relax on following the golden rules that have helped protect Delawareans from getting coronavirus so far.
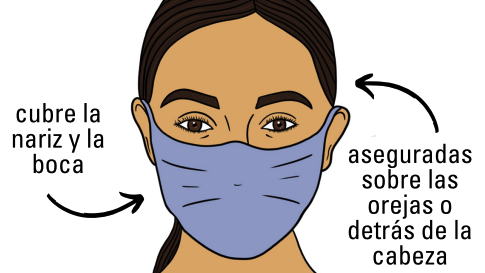
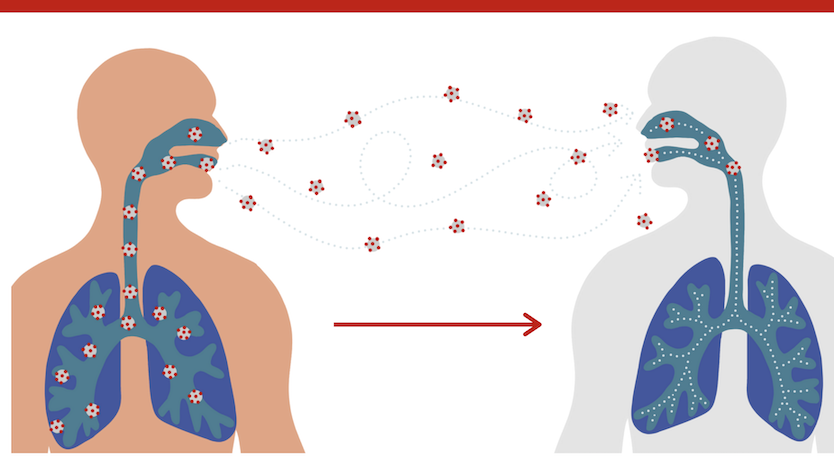
- Masking is recommended.
Practice social distancing.
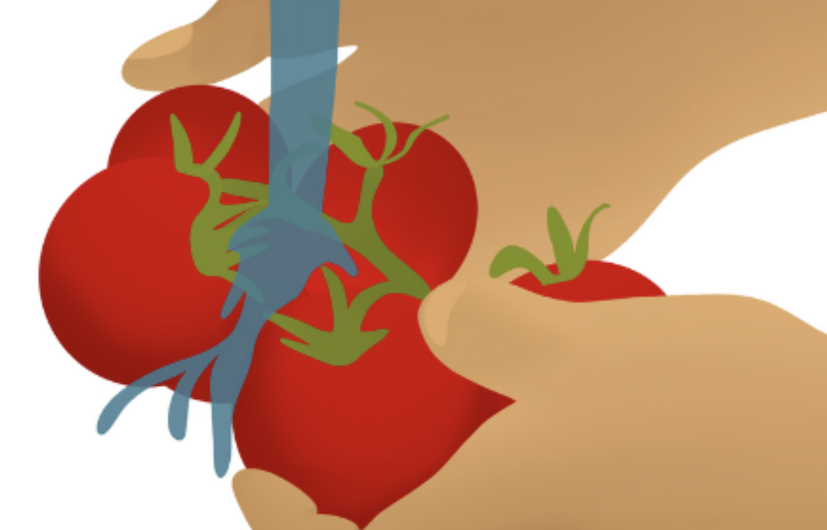
Wash or sanitize your hands frequently, especially after touching surfaces frequently contacted by others.
Stay at home if you feel sick, experience symptoms, or think you have been exposed to COVID-19.
Yes! With both influenza and SARS-CoV-2 circulating, getting both vaccines is important for prevention of severe disease, hospitalization, and death. Getting both vaccines at the same visit increases the chance that a person will be up to date with their vaccinations.
Variants emerge as a result of naturally occurring mutations in viruses (like the yearly flu).
Preliminary data does not suggest that the Omicron variant is causing more severe illness in children.
Omicron variant is more contagious than other variants, but may be less likely to cause severe disease.
The vaccines continue to be highly effective in reducing risk of severe disease, hospitalization, and death.
The CDC recommends that everyone age 5 and older get vaccinated, and everyone age 12 and older get boosted when eligible
If you are...
POSITIVE: The CDC recommends that people who test positive for COVID-19 should stay home and isolate alone and away from others for at least five days after testing positive.
EXPOSED/UNVACCINATED: If you are unvaccinated, or if you are eligible to be boosted and have not yet received your booster, the CDC recommends that you quarantine alone and away from others for five days.
EXPOSED/VACCINATED: If you have been boosted, or have been fully vaccinated and are not yet eligible to be boosted, the CDC recommends that you wear a mask around others for 10 days, but you need not quarantine unless you develop symptoms.
PCR Test:
Most accurate test currently available
Typically administered by health providers at a clinic or pharmacy and analyzed in a laboratory
Results in typically in 24-72 hours
Rapid Antigen Test
Less accurate than PCR tests
Results in as little as 15 minutes when taken at home
Can be self-administered with an at-home testing kit, or taken at a testing site
As of January 15, these tests are covered by health insurance, meaning that most people with health plans will be able to get up to eight (8) tests per month for free through direct coverage or can get reimbursed for purchasing tests by submitting a claim to their insurer.
FLU SEASON
- A few things are different for the 2022-2023 influenza (flu) season, including:
- The composition of flu vaccines has been updated.
- For the 2022-2023 flu season, there are three flu vaccines that are preferentially recommended for people 65 years and older. These are Fluzone High-Dose Quadrivalent vaccine, Flublok Quadrivalent recombinant flu vaccine and Fluad Quadrivalent adjuvanted flu vaccine.
- The recommended timing of vaccination is similar to last season. For most people who need only one dose for the season, September and October are generally good times to get vaccinated. Vaccination in July and August is not recommended for most adults but can be considered for some groups. While ideally it’s recommended to get vaccinated by the end of October, it’s important to know that vaccination after October can still provide protection during the peak of flu season.
- The age indication for the cell culture-based inactivated flu vaccine, Flucelvax Quadrivalent (ccIIV4), changed from 2 years and older to 6 months and older.
- Pre-filled Afluria Quadrivalent flu shots for children are not expected to be available this season. However, children can receive this vaccine from a multidose vial at the recommended dose.
FLU VACCINE AND COVID
Efforts to reduce transmission of SARS-CoV-2 have led to a decrease in routine preventive medical services, including immunization services. Ensuring that routine vaccination is maintained or reinitiated during the COVID-19 pandemic is essential for protecting individuals and communities from vaccine-preventable diseases and outbreaks. Routine vaccination prevents illnesses that lead to unnecessary medical visits and hospitalizations and further strain the healthcare system. For the 2021–2022 influenza season, influenza vaccination will be paramount to reduce the impact of respiratory illnesses attributed to influenza and resulting burdens on the healthcare system during the COVID-19 pandemic. Communicating the importance of vaccination to patients and parents/caregivers, as well as the safety protocols and procedures outlined in this guidance, can help reassure those who may otherwise be hesitant to present for vaccination visits.
Annual influenza vaccination is recommended for all persons aged 6 months and older to decrease morbidity and mortality caused by influenza. Healthcare personnel should consult current influenza vaccine recommendations for guidance around the timing of administration and use of specific vaccines.
During the COVID-19 pandemic, reducing the overall burden of respiratory illnesses is important to protect vulnerable populations at risk for severe illness, the healthcare system, and other critical infrastructure. Thus, healthcare personnel should use every opportunity during the influenza season to administer influenza vaccines to all eligible persons, including:
Essential workers: Healthcare personnel, including staff in post-acute and long-term care facilities, as well as pharmacy staff, and other critical infrastructure workforce
Persons at increased risk for severe illness from COVID-19: Including adults aged 65 years and older, residents in post-acute and long-term care facilities, and persons of all ages with certain underlying medical conditions. In addition, severe illness from COVID-19 disproportionately affects members of certain racial and ethnic minority groups.
Persons at high risk for influenza complications: Including infants aged 6 months and older and young children less than 5 years of age, children with neurologic conditions, pregnant people, adults aged 65 years and older, and other persons with certain underlying medical conditions
It is important to counsel patients about the risk of self-limited side effects after influenza vaccination, including local reactions, such as redness, pain, or swelling at the injection site, and systemic reactions, which include fever, chills, headache, and body aches. If they occur, such side effects normally resolve within 72 hours after vaccination. Because of concerns about COVID-19, if a vaccine recipient develops fever after vaccination, they should stay home until they have been fever-free for 24 hours without the use of fever-reducing medications. Influenza vaccination does not cause respiratory symptoms common in COVID-19, such as cough or shortness of breath. If the vaccine recipient develops new symptoms of COVID-19 (e.g., cough or shortness of breath), or if fever does not resolve within 72 hours of vaccination without the use of fever-reducing medications, the recipient should contact their healthcare provider. If the patient develops emergency warning signs for COVID-19, they should seek emergency medical care immediately.
FLU VACCINE & COVID VACCINE
COVID-19 vaccines may be administered without regard to timing of other vaccines. This includes simultaneous administration of COVID-19 vaccine and other vaccines on the same day. It is not known if the reactogenicity of COVID-19 vaccines is increased with coadministration, including with other vaccines known to be more reactogenic, such as adjuvanted vaccines. When deciding whether to administer an(other) vaccine(s) with a COVID-19 vaccine, vaccination providers should consider whether the patient is behind or at risk of becoming behind on recommended vaccines, their risk of vaccine-preventable disease (e.g., during an outbreak or occupational exposures), and the reactogenicity profile of the vaccines.
If multiple vaccines are administered at a single visit, administer each injection in a different injection site. For adolescents and adults, the deltoid muscle can be used for more than one intramuscular injection administered at different sites in the muscle.
Getting COVID-19 during pregnancy is serious. It can cause you to get very sick and increase the risk of preterm birth (before 37 weeks of pregnancy).
CDC recommends COVID-19 vaccination for all people who are pregnant, breastfeeding, or trying to get pregnant now or in the future
Antibodies made after a pregnant person received an mRNA COVID-19 vaccine have been found in umbilical cord blood, which means that COVID-19 vaccination during pregnancy might help protect babies against COVID-19
There is currently no evidence that shows that any vaccines including COVID-19 vaccines, cause fertility problems in women or men.
The CDC does not know for sure if mothers with COVID-19 can spread the virus to babies in breast milk, but based on what they do know, they claim it is unlikely.