

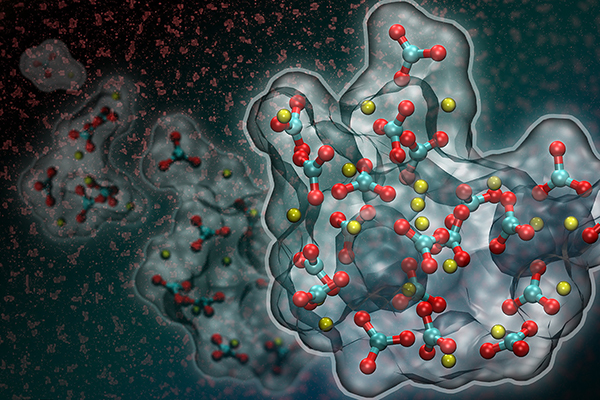
Cultivating crystals
New insight on how crystals form may advance materials, health and basic science research
8:44 a.m., Aug. 5, 2015--Scientists have long worked to understand how crystals grow into complex shapes. Crystals are important in materials from skeletons and shells to soils and semiconductor materials, but much is unknown about how they form.
Now, an international group of researchers that includes the University of Delaware’s Adam Wallace has shown how nature uses a variety of pathways to grow crystals that go beyond the classical, one-atom-at-a-time route.
Research Stories
Chronic wounds
Prof. Heck's legacy
The findings, published July 30 in the journal Science, have implications for decades-old questions in science and technology regarding how animals and plants grow minerals into shapes that have no relation to their original crystal symmetry, and why some contaminants are so difficult to remove from stream sediments and groundwater.
"Researchers across all disciplines have made observations of skeletons and laboratory grown crystals that cannot be explained by traditional theories," said the paper’s corresponding author Patricia Dove, a University Distinguished Professor at Virginia Tech and the C.P. Miles Professor of Science in the College of Science.
"We show how these crystals can be built up into complex structures by attaching particles – as nanocrystals, clusters or droplets – that become organized into complex shapes. Many scientists have contributed to identifying these particles and pathways to becoming a crystal – our challenge was to put together a framework to understand them."
The results emerged from discussions among 15 scientists working in the field of geochemistry, physics, biology, and the earth and materials sciences. The international group met for a three-day workshop in Berkeley, California, that was sponsored by the Council on Geosciences of the Office of Basic Energy Sciences of the U.S. Department of Energy.
By understanding how animals form crystals into the working structures known as shells, teeth and bones, the researchers said scientists will have a bigger toolbox for interpreting the crystals formed in nature.
“As a geologist, understanding how crystals form can help me better understand and interpret ancient sediments, which are records of environmental change. This is important from a fundamental science standpoint, but also as it relates to determining how the planet will respond to environmental perturbations in the future,” said Wallace, an assistant professor in UD’s Department of Geological Sciences, who participated in the review.
In animal and laboratory systems alike, the process of crystal formation begins by developing the particles. They can be small molecules, clusters, droplets, or nanocrystals. These particles are often unstable on their own and facilitate crystal growth by combining with each other and with nearby crystals and surfaces.
For example, nanocrystals prefer to become oriented along the same direction as the larger crystal before attaching, much like adding Legos. In contrast, amorphous conglomerates can simply aggregate. These atoms later become organized by "doing the wave" through the mass to rearrange into a single crystal, researchers said.
The insights may help in the design of novel materials, including photovoltaic thin films and devices for hydrogen storage and carbon capture, and explain unusual mineral patterns in rocks.
Likewise, knowing how pollutants are transported or trapped in the minerals of sediments has implications for environmental management of water and soil.
While the study evidence shows that these pathways to growing a crystal become possible because of interplays between of thermodynamic and kinetic factors, the study authors say more work is needed to understand the forces that cause these particles to move and combine.
The work was supported by the Council on Geosciences of the U.S. Department of Energy, Office of Basic Energy Sciences, Chemical Sciences, Geosciences and Biosciences Division.
About the professor
Adam Wallace joined the University of Delaware in 2013 as an assistant professor in the Department of Geological Sciences, which is housed in the College of Earth, Ocean, and Environment.
His research explores how biological interactions influence the formation, growth, assembly and properties of biominerals, which are the primary record of life and environmental change on Earth.
Wallace earned a bachelor of science in geology at University of California in 2001 and a doctoral degree in geochemistry from Virginia Polytechnic Institute and State University in 2008. Prior to joining UD, he was a post-doctoral fellow in the Earth Science Division and Molecular Foundry at Lawrence Berkeley National Laboratory from 2009-13.
Image by Adam Wallace, University of Delaware, and David J. Carey
Photo by Kathy F. Atkinson









