The
Dybowski Research Group
Materials Characterization
Group Members
|
Cecil Dybowski Ph.D., University of Texas at Austin |
Shi Bai Ph.D., Brigham Young University |
B. S., University of Pittsburgh |
Research in the Dybowski research group provides insight into the properties of solid and semi-solid materials. Much of the early work has been NMR analysis of materials through studies of magnetic shielding and relaxation. More recently, we have used other characterization tools such as FTIR and X-ray analysis, specifically synchrotron X-ray analysis. The information provides a basis for understanding the structure and function in these complex systems. The studies include computational chemistry to predict spectroscopic properties of materials, usually with density functional theory.
With our long-standing interest in the topic, we develop the theory of NMR spin-lattice relaxation in solids, particularly materials containing heavy nuclei. Most theoretical studies in our group, such as the unusual relaxation mechanism in Pb-207 in solids, are motivated by unusual experimental results.
Materials
at Surfaces
Early work on materials at surfaces centered on identifying species formed during heterogeneous catalysis or interaction with a surface.
1. The observation of a hydride-like species on the surface of a supported-rhodium catalyst by adsorption of hydrogen, and how it interacts with carbon monoxide
2. Structures formed when various osmium carbonyl compounds are put in contact with magnesium oxide.
3. The structure and dynamics of adsorbed methanol on ZSM-5 catalysts, both immediately after adsorption, and following heating to promote reaction.
4. The NMR spectroscopy of xenon gas sorbed in various zeolites delineates the nature of xenon-zeolite, xenon-xenon and xenon-coadsorbate interactions.
5. Study of ion-molecule complexes in zeolites and exchange with free organic materials.
6. ESR studies of radicals formed at the surface of supported-metal catalysts.
Our laboratory rarely studies surface reactions at the present time.
Experimental NMR
Chemical Shifts of Spin-1/2 Heavy Metals in Solids
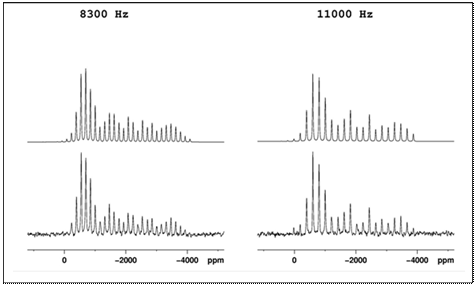 Almost every element has at least
one isotope amenable to investigation with NMR spectroscopy. Our group has studied, both experimentally
and theoretically, the chemical-shift tensors of several heavy-metal spin-˝ nuclei
in solid materials, 207Pb, 119Sn, 113Cd, and 199Hg.
Example magic-angle-spinning 199Hg NMR spectra (predicted and
experimental) of HgCl are shown below at two
different spinning speeds. The table
shows Pb-207 data for various pure lead-containing solids determined by our
group from spectra such as those in the figure. Measurements show that, for example, Pb-207
NMR parameters are strongly affected by the local environment of the lead
ion. Complexation
of lead ion by materials such as 1,10-phenanthroline and thiourea,
for example, alters the NMR chemical-shift parameters, which can be used to
specify lead co-ordination in solids.
Almost every element has at least
one isotope amenable to investigation with NMR spectroscopy. Our group has studied, both experimentally
and theoretically, the chemical-shift tensors of several heavy-metal spin-˝ nuclei
in solid materials, 207Pb, 119Sn, 113Cd, and 199Hg.
Example magic-angle-spinning 199Hg NMR spectra (predicted and
experimental) of HgCl are shown below at two
different spinning speeds. The table
shows Pb-207 data for various pure lead-containing solids determined by our
group from spectra such as those in the figure. Measurements show that, for example, Pb-207
NMR parameters are strongly affected by the local environment of the lead
ion. Complexation
of lead ion by materials such as 1,10-phenanthroline and thiourea,
for example, alters the NMR chemical-shift parameters, which can be used to
specify lead co-ordination in solids.
Investigation of Nuclei with Spin Greater than 1/2
![Text Box: Lead Chemical-Shift Parameters of Solid Materials
Compound Isotropic Shift [ppm] Span [ppm] Skew
Lead sulfate -3608 566 0.37
Lead nitrate -3491 54 1.00
Lead selenate -2849 512 -1.00
Lead niobate -2844 221 -0.70
Lead meta-tantalate -2724 510 -1.00
alpha-Lead fluoride -2666 470 0.58
Lead carbonate -2622 764 0.56
Lead sulfite -2463 446 0.80
Lead meta-vanadate -2295 517 -0.15
Lead chromate -2284 884 -0.10
Lead molybdate -2012 182 -1.00
Lead tungstate -2003 198 -1.00
Lead chloride -1715 533 0.52
Lead thiocyanate -1593 1275 -0.58
Lead titanate -1409 1139 1.00
Lead zirconate (a) -1401 860 0.65
Minium, Pb3O4 (Pb4+) -1091 123 1.00
Lead zirconate (b) -1055 1172 0.95
Lead bromide -981 699 0.58
Lead hydroxychloride -706 2341 0.57
Lead hydroxybromide -639 2101 0.63
Lead hydroxyiodide -546 1726 0.83
Lead iodide -29 0.0 0.00
Lead sulfide 113 0.0 0.00
Minium, Pb3O4 (Pb2+) 804 3088 0.72
beta-Lead oxide 1527 3820 0.97
alpha-Lead oxide 1930 3300 1.00
PbO2 5206. 2473 0.05](research_files/image009.gif) Spins like 129I and 43Ca
are quadrupolar, and as such are sensitive to the electric-field gradient at
the site of the nucleus. Quadrupolar
parameters can be used to define the local electronic
Spins like 129I and 43Ca
are quadrupolar, and as such are sensitive to the electric-field gradient at
the site of the nucleus. Quadrupolar
parameters can be used to define the local electronic 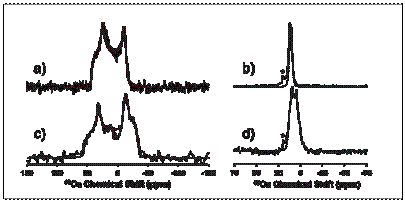 environment in
materials that contain centers like Ca.
For example, many active pharmaceutical ingredients are often calcium
salts, and study of the 43Ca NMR spectroscopy may be useful in
distinguishing different sites or different phases that form in such solids.
For example, in the figure are shown 43Ca spectra of atorvastatin
calcium taken at two field strengths and with and without magic-angle
spinning. From analysis of the line
shapes (shown in color), one can easily determine the quadrupolar parameters of
the site at which the calcium nucleus resides, which specifies properties of
the electronic structure.
environment in
materials that contain centers like Ca.
For example, many active pharmaceutical ingredients are often calcium
salts, and study of the 43Ca NMR spectroscopy may be useful in
distinguishing different sites or different phases that form in such solids.
For example, in the figure are shown 43Ca spectra of atorvastatin
calcium taken at two field strengths and with and without magic-angle
spinning. From analysis of the line
shapes (shown in color), one can easily determine the quadrupolar parameters of
the site at which the calcium nucleus resides, which specifies properties of
the electronic structure.
Temperature-dependent
Pb-207 Chemical Shifts in Solids
In many lead-containing solids, the chemical shift is strongly temperature-dependent. This characteristic, for examle, provides a means of determining the temperature in the NMR probe directly. We have demonstrated means to use this phenomenon as a thermometer in NMR spectroscopic experiments. For example, the temperature variation of the “peak” of a Pb(NO3)2 powder pattern is given by the simple equation.
dPb (T) = - {3670.6 +/- 1.0) ppm + {0.666 +/- 0.003 ppm/K} T
The origin of the temperature variation has been investigated. We have correlated the temperature dependence of the chemical-shift tensor elements with thermal expansion of the lattice, to show that the observed NMR changes are evidence of the dependence of electronic state on structural properties such as the unit-cell dimension. One may derive, for example, a prediction of the NMR properties as a function of the unit cell dimension that connects these two parameter theoretically, through the variation of the solid-state wave function by a perturbation expansion.
The variation with
temperature is not unique to the 207Pb resonance. For example, our recent measurement of the
temperature variation of the chemical shift of tetraethylammonium sodium
tetracyanomercurate show that the isotropic position of the 199Hg
resonance varies linearly with temperature from room temperature to 50oC
in the following manner
dHg (T) = -
381.01 ppm - {0.1781 ppm/K} T
Theory of
Relaxation in Heavy-Metal Systems
 NMR
relaxation reflects molecular dynamics around the nuclear spin’s site. By
conventional theory, it has long been known that random rotational dynamics
induce spin-lattice relaxation of spins, as has been known since the late
1940s. The nature and efficiency of
spin-lattice relaxation depends on the mechanism of coupling with other degrees
of freedom of the material. For spin-1/2
nuclei there are numerous mechanisms by which coupling may allow energy
transfer.
NMR
relaxation reflects molecular dynamics around the nuclear spin’s site. By
conventional theory, it has long been known that random rotational dynamics
induce spin-lattice relaxation of spins, as has been known since the late
1940s. The nature and efficiency of
spin-lattice relaxation depends on the mechanism of coupling with other degrees
of freedom of the material. For spin-1/2
nuclei there are numerous mechanisms by which coupling may allow energy
transfer.
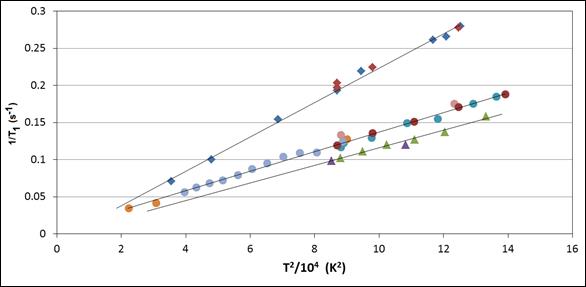
We discovered unusual experimental T1 dependences on temperature and field in several pure lead-containing materials (shown in the figure). In each case, the result was
1/T1 a T2
with no dependence on magnetic field, as seen in the figure for Pb(NO3)2, PbMoO4 and PbCl2. This form is unusual, and is not what one might predict by “normal” or “standard” theories of relaxation which assume random isotropic rotational motion to modulate the coupling of spins with their surroundings.
We have developed a semiclassical theory of relaxation that explains these observations. The model upon which the theory is based involves interaction with phonons through modulation of the spin-rotation coupling. In addition to Pb-207 relaxation, the mechanism has also been experimentally seen for tin in SnF2 at sufficiently low temperature, for thallium in certain compounds, and – recently in our laboratory – for Hg-containing solids. Interestingly, this mechanism does not seem to be a major mechanism for the relaxation of 113Cd in CdMoO4, for which relaxation by interaction with remote paramagnetic centers seems to dominate the relaxation behavior. The theory predicts that the mechanism should only be effective for materials with large spin-rotation coupling constants, as is often the case for heavy nuclei.
We also have a strong interest in understanding the nature of spin-lattice relaxation in solids such as semiconductors, where several competing processes may exert strong effects on it.
Computational
Prediction of Magnetic Shielding of Heavy Nuclei in Solids
 Magnetic
shielding is the major characteristic of NMR spectra that is useful in
identifying nuclear sites. The shielding
arises from coupling to the magnetic fields produced by electrons. Thus, it gives an indirect measure of the
electronic states. We use density
functional theory to calculate electronic states and thereby predict the
magnetic shielding of nuclei in solids.
To predict the shielding of heavy nuclei such as Pb-207 and Hg-199 in
solids requires one to confront issues not as important for light nuclei. First, electrons associated with heavy nuclei
must be treated as relativistic particles.
Second, in the solid state, the structures formed are often not “molecular”
in the sense that a simple organic structure is. Thus, calculation of electronic properties
such as the magnetic shielding requires one to consider the extended structure
of the solid. In our laboratory, we
predict the full chemical shielding tensor of nuclei like Pb-207 and Hg-199
using density functional theory augmented with the zero order regular
approximation (ZORA) to take into account (approximately) the relativistic
nature of the electrons. The calculations
to connect NMR parameters with structure require the use of cluster models of
the solid structure (to be manageable for calculation) developed in our
laboratory. The studies have resulted in
a systematic method for calculation of NMR parameters when a structure is known
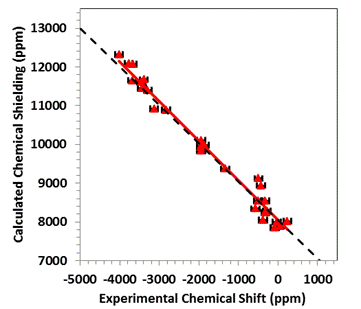
that achieves agreement with experiment to within about 2%. For example, in the figure the calculated
principal components of the magnetic-shielding tensors of a suite of
Hg-containing materials are correlated with the experimentally measured
chemical shifts. The dashed line is the
trend that should be followed if the calculation absolutely reproduces the
experimental data. The red line is the
best-fit linear correlation between these two sets of parameters. As one can see, the experimental and
calculated quantities agree within experimental error.
Magnetic
shielding is the major characteristic of NMR spectra that is useful in
identifying nuclear sites. The shielding
arises from coupling to the magnetic fields produced by electrons. Thus, it gives an indirect measure of the
electronic states. We use density
functional theory to calculate electronic states and thereby predict the
magnetic shielding of nuclei in solids.
To predict the shielding of heavy nuclei such as Pb-207 and Hg-199 in
solids requires one to confront issues not as important for light nuclei. First, electrons associated with heavy nuclei
must be treated as relativistic particles.
Second, in the solid state, the structures formed are often not “molecular”
in the sense that a simple organic structure is. Thus, calculation of electronic properties
such as the magnetic shielding requires one to consider the extended structure
of the solid. In our laboratory, we
predict the full chemical shielding tensor of nuclei like Pb-207 and Hg-199
using density functional theory augmented with the zero order regular
approximation (ZORA) to take into account (approximately) the relativistic
nature of the electrons. The calculations
to connect NMR parameters with structure require the use of cluster models of
the solid structure (to be manageable for calculation) developed in our
laboratory. The studies have resulted in
a systematic method for calculation of NMR parameters when a structure is known
that achieves agreement with experiment to within about 2%. For example, in the figure the calculated
principal components of the magnetic-shielding tensors of a suite of
Hg-containing materials are correlated with the experimentally measured
chemical shifts. The dashed line is the
trend that should be followed if the calculation absolutely reproduces the
experimental data. The red line is the
best-fit linear correlation between these two sets of parameters. As one can see, the experimental and
calculated quantities agree within experimental error.
The results suggest that, with current available computational resources and reasonably large cluster models, one can fairly quantitatively predict NMR parameters of these heavy nuclei. Network solids require special considerations in constructing the clusters to ensure agreement with experiment. We have developed models to allow these calculations for both molecular and network solids.
Computational Prediction of
Magnetic Shielding for Organic Solids
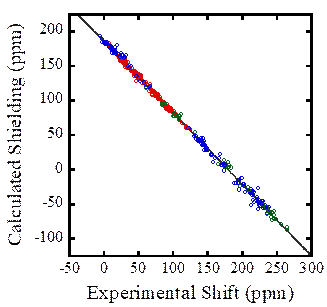 The
C-13 magnetic shielding of nuclei in solid phases may also be predicted from
approximation of the solid structure with cluster models and density functional
theory. For light elements like carbon,
the effects of relativity are usually minimal (although this must always be
checked), which simplifies the computational methodology for these
materials. We have investigated a wide
variety of carbon resonances representing various electronic environments. The use of appropriate large-cluster models
results in quantitative agreement with experimentally determined NMR chemical
shifts, as seen in the figure. The different colors represent carbons in
various types of environments: aliphatic, aromatic, and carboxyl
environments. Note that all types of
carbon obey a single relationship between calculated and experimental
parameters. A comparison of these data
to predicted shieldings from an isolated-molecule model demonstrates that,
although a great deal of the chemical shielding for organic materials arises
from electrons associated with the molecule in which the nucleus resides, the
effects of other molecules cannot be neglected in prediction of solid-state
shielding effects. As with the
heavy-nucleus-containing materials, clusters having appropriate symmetry
elements and sufficiently large size are required to ensure agreement with
experiment. Typically, the appropriate
model cluster contains greater than 13-15 molecules, appropriately chosen so as
to represent the environment.
The
C-13 magnetic shielding of nuclei in solid phases may also be predicted from
approximation of the solid structure with cluster models and density functional
theory. For light elements like carbon,
the effects of relativity are usually minimal (although this must always be
checked), which simplifies the computational methodology for these
materials. We have investigated a wide
variety of carbon resonances representing various electronic environments. The use of appropriate large-cluster models
results in quantitative agreement with experimentally determined NMR chemical
shifts, as seen in the figure. The different colors represent carbons in
various types of environments: aliphatic, aromatic, and carboxyl
environments. Note that all types of
carbon obey a single relationship between calculated and experimental
parameters. A comparison of these data
to predicted shieldings from an isolated-molecule model demonstrates that,
although a great deal of the chemical shielding for organic materials arises
from electrons associated with the molecule in which the nucleus resides, the
effects of other molecules cannot be neglected in prediction of solid-state
shielding effects. As with the
heavy-nucleus-containing materials, clusters having appropriate symmetry
elements and sufficiently large size are required to ensure agreement with
experiment. Typically, the appropriate
model cluster contains greater than 13-15 molecules, appropriately chosen so as
to represent the environment.
In the limit of large clusters, the mean error in calculation of tensor components is less than 2 ppm, sufficient to allow calculational results to aid in assignment of spectra.
 Studies
of the Formation of Lead Soaps in Masterworks of Art
Studies
of the Formation of Lead Soaps in Masterworks of Art
Lead soaps, salts of lead ions with fatty acids, are frequently found in paintings from the 14th through the 20th century. Over time, reactions between free fatty acids in the binder and lead (or other ions) from pigments produce these unwanted materials, and degrade the paintings. In some cases, there is the formation of protrusions, or the creation of areas of transparency. In joint studies with the Metropolitan Museum of Art, we have begun to use NMR, FTIR, and other techniques to study these effects in model paint systems, in the hope that our analyses may lead to a better understanding of the chemistry, thereby permitting the development of measures to stop of slow down these reactions on these extremely valuable cultural artifacts. Recently, we have extended the studies to include synchrotron X-ray techniques (such as XANES and XRF).
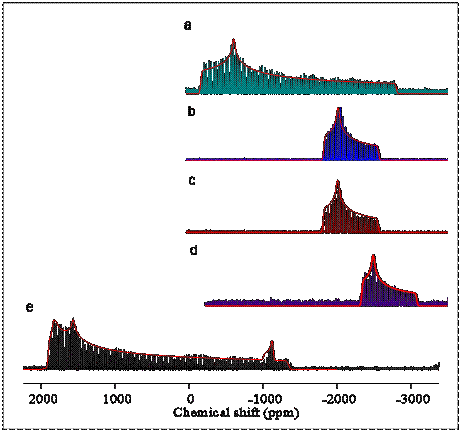
Pb-207 spectroscopy of these model materials is extremely challenging for the spectroscopists because of the very wide resonance lines in the spectrum. Spectra of several pure materials obtained with the so-called WURST-CPMG NMR technique show a “spikelet” spectrum which mimics the continuous Pb-207 band shape of the material. (See the figure.) After an initial phase of identifying the lead signatures of possible materials in a film, we have begun studying paint films that model the slow chemistry in such paintings. Spectrum (e) is of the lead-tin yellow type I pigment, which shows the presence of impurities. Spectra (a) – (c) are of various lead fatty-acid esters. Spectrum (d) is of lead carbonate, an impurity in lead white, a common paint pigment. The lead-tin yellow type I pigment has two unique sites for lead. The spectrum of lead tin yellow, type I, also shows an impurity of minium, the starting material for making the pigment, at the level of a few percent.
Studies in our laboratory show that reaction to produce lead soaps occur when free acid is allowed to contact paint films. The process in such a complex system has many parts. Currently, we are studying the diffusion of free acids in paint films, and the porosity and tortuosity of those films, as those factors affect the reaction.
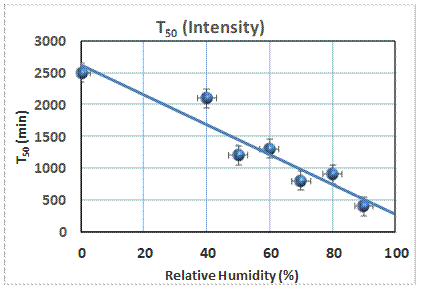 Reaction
to produce soaps in painting by subjecting the material to 13C-enriched
materials such as palmitic acid, one of the suggested precursors. By monitoring the 13C NMR
spectrum, the kinetics of the formation of the soap is easily
characterized. The reaction is complex,
and is characterized by the time, T50, at which half of the signal
corresponds to the soap. This measure
depends on the relative humidity to which the sample has been exposed, as seen
in the figure. This is consistent with
the notion that the transport of the reactants is influenced by the movement of
water in these materials.
Reaction
to produce soaps in painting by subjecting the material to 13C-enriched
materials such as palmitic acid, one of the suggested precursors. By monitoring the 13C NMR
spectrum, the kinetics of the formation of the soap is easily
characterized. The reaction is complex,
and is characterized by the time, T50, at which half of the signal
corresponds to the soap. This measure
depends on the relative humidity to which the sample has been exposed, as seen
in the figure. This is consistent with
the notion that the transport of the reactants is influenced by the movement of
water in these materials.
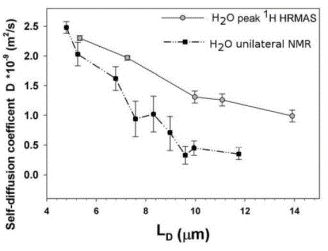 The
dynamics of water in these paint films is probed by measurement of the
diffusion coefficient of water using unilateral NMR techniques. One may probe the diffusion on various length
scales, as indicated in the figure, where LD is the diffusion length
scale. For sufficiently short length
scale, the diffusion of water in these materials is equivalent to the diffusion
in bulk water, but for longer diffusion lengths, the shorter apparent diffusion
coefficients indicate that the diffusion is bounded, as expected for diffusion
in finite-size spaces. (Measurements by two different methods give slightly
different values, but the trend is the same.)
The behavior in these experiments suggests that the porosity accessible
to water in these materials is ~6%.
The
dynamics of water in these paint films is probed by measurement of the
diffusion coefficient of water using unilateral NMR techniques. One may probe the diffusion on various length
scales, as indicated in the figure, where LD is the diffusion length
scale. For sufficiently short length
scale, the diffusion of water in these materials is equivalent to the diffusion
in bulk water, but for longer diffusion lengths, the shorter apparent diffusion
coefficients indicate that the diffusion is bounded, as expected for diffusion
in finite-size spaces. (Measurements by two different methods give slightly
different values, but the trend is the same.)
The behavior in these experiments suggests that the porosity accessible
to water in these materials is ~6%.
Such experiments give information on the nature of the steps in the reactive process to produce soaps.
![]() Cecil Dybowski,
1998 - 2020.
Cecil Dybowski,
1998 - 2020.
Last Updated: January 21, 2020
URL of this document: http://www.udel.edu/dybowski/research.htm


