Perspectives on Pyridine-Nucleotide-Dependent Dehydrogenases
NAD (Nicotinamide-Adenine Dinucleotide) and its phosphorylated derivative, NADP, serve as coenzymes for pyridine nucleotide-dependent dehydrogenases. Approximately one sixth of all enzymes involved in intermediary metabolism require a pyridine nucleotide coenzyme. While NAD and NADP have similar chemical properties, dehydrogenase enzymes are specific for one or the other with very few exceptions. The following problems examine reactions catalyzed by four dehydrogenases from the core of metabolism.
1. Malic enzyme catalyzes a reaction analogous to the isocitrate dehydrogenase reaction. It is quite specific for NADP+.
a) What compound would you predict to be an enzyme-bound intermediate in this reaction? Show how your proposed intermediate would breakdown to give products. Indicate how the electrons would move. What functional groups on the enzyme might facilitate this breakdown?
b) Isocitrate dehydrogenase in the presence of NADPH can slowly reduce the compound which is the intermediate in the malic enzyme reaction. The product of this reduction behaves chemically like malate but is not a substrate for malic enzyme. Explain.
c) Malate dehydrogenase, an enzyme in the citric acid cycle, reversibly converts L-malate to oxaloacetate using NAD+. NADP+ will not substitute for NAD+. If both malic enzyme and malate dehydrogenase are incubated together with L-malate and equal amounts of NAD+ and NADP+ and the reaction mixture assayed for products when the 340 nm absorbance ceases to increase, how would the NAD+/NADH ratio compare to the NADP+/NADPH ratio? Does your answer seem consistent with the fact that the redox potential (Eo' at pH 7.0) for both pyridine nucleotide couples is -0.32 v? (General information: Eo' at pH 7.0 for pyruvate + CO2/malate = -0.33 v; for oxaloacetate/malate, -0.175 v). Can any generalization be made about reactions producing NADPH as opposed to those generating NADH?
d) Near neutrality, is either the malic enzyme equilibrium or the malate dehydrogenase equilibrium sensitive to changes in pH? Explain your answer.
2. L-lactate dehydrogenase (LDH) is A-specific with respect to the hydrogen at the C-4 position of the pyridine ring of NAD. That is, it will transfer only the HA hydrogen to pyruvate to form lactate.
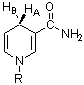 |
D-glyceraldehyde-3-P dehydrogenase (GAPDH) is specific for the B-position. GAPDH normally forms 1,3-diphosphoglycerate when phosphate is present. Arsenate will substitute for phosphate in the reaction but the acyl arsenate formed quickly hydrolyzes to regenerate arsenate and form 3-phosphoglycerate. |
b) In an assay of GAPDH, why would there be an advantage to using arsenate rather than phosphate?
c) Glyceraldehyde-3-P labeled with deuterium (2H)
on the carbonyl carbon is incubated with NAD+, arsenate, pyruvate,
GAPDH, and LDH. Follow the transfer of deuterium in the reaction mixture.
Will 2-2H-lactate
be formed in the first turn over of NAD2H?,
the second?, ever? What would be the answer if GAPDH were A-specific?