There are 10 pages to this half of the examination. Write your name on each new page. Read every question so that you understand what is being asked. If you feel any question is unclear or ambiguous, clearly explain your answer or interpretation. Please call my attention to any errors you encounter.
The final two pages of this examination include a copy of the universal genetic code and a sheet with metabolic pathways, respectively. You may remove them for reference.
This examination will assess your learning, problem-solving skills, and ability to communicate clearly. Parts are intended to be challenging even to the best students in the class. Some of the questions will deal with material you have not seen before and is not in your text; however, they can be answered by applying basic principles discussed in the course.
Do not expose your answers to the scrutiny of your neighbors. Please fold under each page before you go on to the next.
Breakdown of the examination by sections:
Structures
16 Points
Problems
59 Points (+2 Bonus)
Short Essay
10 Points
Total
85 Points
Exam Statistics:
N 40
Range 17 – 77.5
Mean 49.1 ± 12.9

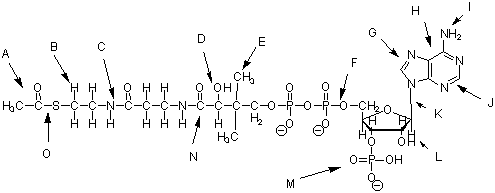
Name of this compound ______________________________
A _______________ Two carbon group I ________________ the NH2 group
B _______________ -CH2- group J ________________ the 6-member ring
C _______________ the C-N bond K ________________ the C-N bond
D ________________ the -OH group L ________________ the 5-carbon sugar
E ________________ the -CH3 group M ________________ the -PO3- group
F ________________ the P-O bond N ________________ the C=O group
G ________________ the 5-member ring O ________________ the C-S bond
H ________________ the bicyclic ring
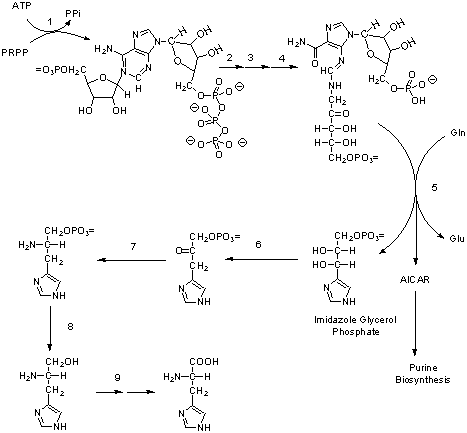
A. (2 points) ATP is a precursor of histidine. In the figure above, draw circles around each atom in histidine that is derived from ATP.
B. (3 points) Examine reaction 5 and draw the expected structure for AICAR to the right of the figure.
C. (16 points) Fill in the following table. Be specific.
If there are no other substrates, products, or cofactors, say "none."
|
|
|
|
|
|
|
|
||||
|
|
||||
|
|
||||
|
|
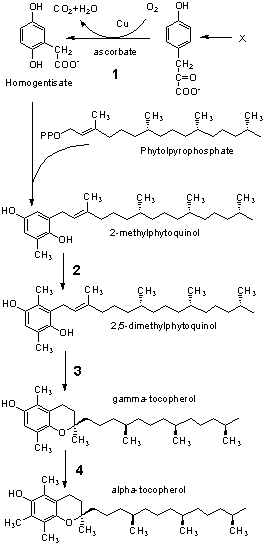 |
A. (1 point) Of the enzyme reactions shown, only 1 occurs
in humans and is part of the catabolic pathway for an amino acid. What
amino acid would that be the direct precursor?
____________________
B. (1 point) Name a precursor of phytolpyrophosphate. _____________________
C. (1 point) What would be the expected methyl donor in reactions 2 and 4? ______________________
D. (5 points) Assuming that the same transgenic manipulations can be done in a commercial crop, what biochemical or nutritional concerns would you have before going into production for human consumption? |
i. This question will focus on the exquisite fine control known as attenuation which couples transcription to translation. The following are some of the features of the attenuation mechanism quoted or paraphrased from Keller & Calvo [PNAS, 76, 6186-90 (1979)].Most transcription initiated at the relevant promotor terminates before the structural genes of the operon are reached, resulting in the synthesis of a leader RNA of about 150 nucleotides.
ii. The site at which termination occurs ("attenuator") is similar to previously identified transcription termination sites (rho-independent termination sites). It is a palindromic G-C rich region followed by a series of adenosines on the coding strand. The corresponding region of the leader RNA, which has a potential stem and loop structure followed by a series of uridines, is called the "terminator."
iii. Each of the known leader RNAs contains a second potential stem-and-loop structure proximal to the terminator and overlapping with it in such a way that pairing with one region precludes pairing with the other. (The complimentary palindromic regions that form stems may have a sizable intervening non-complimentary loop.)
iv. Within each leader RNA, translational start and stop signals are positioned so that a peptide of 14-28 amino acids might be synthesized.
iv. Each leader peptide contains in high frequency the amino acid corresponding to the particular operon.
v. The derepression of the operon requires the transcriptional read-through of the attenuator. This occurs only if a ribosome initiates the synthesis of the leader peptide and is retarded in its progress by lower than normal amounts of a specific amino acyl tRNA. This favors a different conformation of the leader RNA and signals transcriptional read through.
The DNA sequence on the next page corresponds to the control
region of a particular amino acid operon from Salmonella typhimurium [PNAS
75(9), 4281 (1978)]. Like mRNA, it is complementary to the template strand.
A genetic code sheet is provided at the end of this examination for you
assistance in some of the questions that follow.
5’...TAAGCATTCATCGGAATTTTTATGACACGCGTTCAATTTAAACACCACCATCATCACCATCATCCTGACTAGTCTTTCAGGCGATGTGTGCTGGAAGACATTCAGATCTTCCAGTGGTGCATGAACGCATGAGAAAGCCCCCGGAAGATCA
TCTTCCGGGGGCTTTTTTTTTGGCGCGCGATACAGACCGGTTCAGACAGGATAAAGAGGAACGCAGAATGTTAGACAA
CACCCGCTTACGCATAGCTATTCAGAAAT...3'
A. (1 Point) Underline the transcriptional termination
region.
B. (2 Points) This DNA sequence (sense strand) corresponds to that of the relevant leader RNA and mRNA. Circle the initiation and termination codons of the leader peptide.
C. (2 Point) Put a box around the initiation codon for the first structural gene.
D. (3 Points) Write the predicted amino acid sequence of the leader peptide above the appropriate codons. (2 point bonus if you can use the correct one letter representations for the amino acids.)
E. (2 Points) This sequence is derived from the control region of the operon for what amino acid?
F. (2 Points) Identify the palindromic region corresponding to the terminator with opposing arrows drawn above the sequences. (Note that the palindromic properties refer to double-stranded DNA of which only one strand is shown. Each strand in these regions have the potential to form stem-loop structures where the loops are unpaired.)
G. (2 Points) Identify the second palindromic region which overlaps the terminator with opposing arrows drawn under the sequences.
H. (4 Points) In the space below, depict in a general way the alternative base-paired structures possible for the leader RNA corresponding to the above sequence of DNA. Which conformation would be favored by low levels of the relevant amino acid?
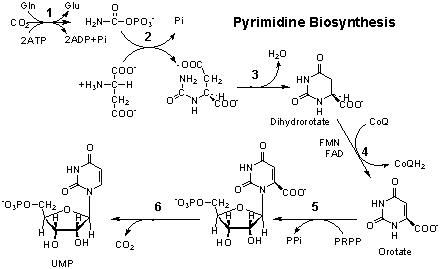
A. (2 points) Reaction 3 involves a dehydration but does
not use ATP. Explain.
B. (2 points) In bacteria, reaction 4 is specific for
NAD, not NADP. Explain.
C. (2 points) Which enzyme reaction would you predict
to regulate the flux in this pathway? What molecules would activate or
inhibit this enzyme?
D. (5 point bonus) Suggest a reasonable explanation for
the orotate overproduction in young rats deprived of arginine.
B. (10 points) Draw a diagram depicting the world record running speeds as a function of the log of the time of running and explain the metabolic differences in muscle metabolism for sprinters, middle distance, and long distance runners.