10 AM to noon, Saturday, 9 March 2002
Dr. Hal White - Instructor
There are 13 pages to this examination including this page and the metabolic pathway sheet at the end. You can remove the last page for handy reference. Write your name on each new page. Read every question so that you understand what is being asked. If you feel any question is unclear or ambiguous, clearly explain your answer or interpretation. Please call my attention to any errors you encounter.This examination will assess your learning, problem-solving skills, and ability to communicate clearly. It is intended to be challenging even to the best students in the class. Some of the questions will deal with material you have not seen before and is not in your text; however, they can be answered by applying basic principles discussed in the course.
Do not expose your answers to the scrutiny of your neighbors. Please fold under each page before you go on to the next.
Breakdown of the examination by sections:
I. Short Answer 15 Points
II. Structure 16 Points
III. Multiple choice 18 Points (+5 Bonus)
IV. Problems 38 Points (+2 Bonus)
V. Short Essays 20 Points
Total 107 Points (+7 Bonus)Exam Statistics:
N = 41 Range 33 - 111/114 Mean 60.3 ± 16.7
__________________________ 2. Another keto acid common intermediate.
__________________________ 3. And the third keto acid common intermediate.
__________________________ 4. In the absence of
oxygen, the number of ATP's
obtainable from NADH in glycolysis.
__________________________ 5. Highly regulated dodecameric
enzyme involved in
nitrogen metabolism.
__________________________ 6. Approximate half-life of an ATP molecule in the human body.
__________________________ 7. Common element in
biochemical compounds that lacks a
radioactive isotope for tracer studies.
__________________________ 8. PI (not pI or Pi) in biochemistry stands for ___.
__________________________ 9. A fatty acid with
the designation C18:2 is used in the
manufacture of what common floor-covering material.
__________________________ 10. Six-carbon thioester intermediate
in leucine catabolism,
steroid synthesis, and ketone body formation.
__________________________ 11. Crop plant genetically engineered to produce ??carotene in its seeds.
__________________________ 12. Associated with "acetone breath."
__________________________ 13. The "G" in heterotrimeric G-proteins stands for?
__________________________ 14. Molybdenum is associated with this enzyme.
__________________________ 15. Value for "adenylate
energy charge," if only ADP
were present in a cell.
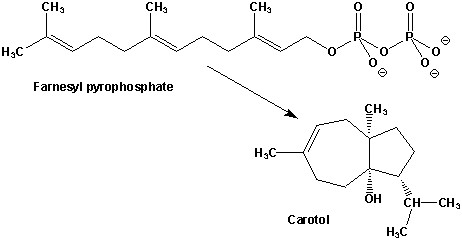
2. (5 Points) Carrot seeds contain carotol, a sequiterpene derived from farnesyl pyrophosphate (FPP) as indicated in the figure below.

A. (3 points) Assuming that none of the carbon-carbon bonds in FPP are broken in the conversion, clearly mark the new carbon-carbon bond(s) in carotol.
B. (2 points) Draw a circle around the carbon in carotol
that is derived from the oxygen bearing carbon of FPP.
3. (8 points) Each year many people die due to organ failure
while waiting for a human organ donor. Survival is not guaranteed after
organ transplant because immunological rejection can occur. Oligosaccharides
attached to membrane lipids and cell surface proteins form antigens (epitopes),
such as the A, B, and O blood groups that play a key role in the rejection
reaction. Because pigs are similar in size to humans, have similar metabolism,
and are already raised for human use, medical researchers have begun to
genetically modify pigs to produce "humanized" organs that evade the rejection
process. The Feb 8 issue of Science [295, 1089 (2002)] reports a "knockout"
pig lacking a gene for ?-1,3-galactosyltransferase, an enzyme that attaches
galactose to cell surface oligosaccharide to generate pig-specific epitopes.
Assume that the enzyme transfers the galactosyl unit to the N-acetyl glucosamine
(GlcNAc) unit shown below.

A. (5 Points) Draw in the structure of galactose as it would be attached by the enzyme to the GlcNAc unit above.
B. (3 Points) Based on analogy to the formation of glycosydic
bonds elsewhere, make a reasonable prediction for the structure of the
galactosyl substrate for this reaction in the space below.
The following multiple choice questions are based on home work problem sets. Please feel free to provide a narrative explanation of your answers if you want to justify your answer.
_____ 1. KDPG Aldolase catalyzes the formation of
pyruvate and glyceraldehyde 3-phosphate.
Knowing that this enzyme has an active site lysine residue but not knowing
which
_____ 2. lysine among many it was, you propose
an experiment to specifically and irreversibly
label the active site lysine. Which two labeled compounds among
the following would
not be part of a reasonable strategy?
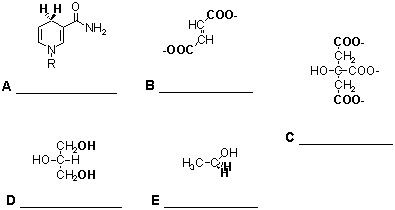
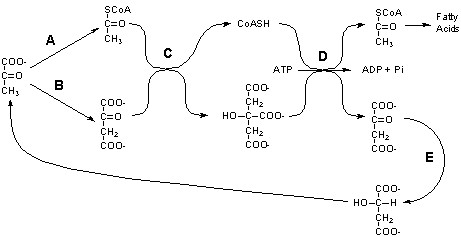
_____ 3. The following metabolic intermediates have elements of symmetry in their structure that are highlighted in bold letters. Identify the one compound in this group for which an enzyme could not distinguish between the two highlighted functional groups. For one bonus point each, name each intermediate in the space provided.A. 15N-Lysine
B. 14C-Pyruvate
C. NaB3H4
D. 14C-Glyceraldehyde 3-P
E. 14C-KDPG
_____ 5. Which of the branched chain fatty acids below
did not have acetyl CoA as a
precursor to its biosynthesis?
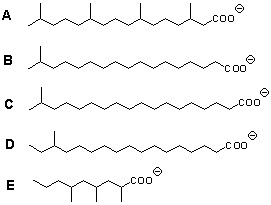
_____ 6. Which of the branched chain fatty acids above
has a precursor most closely related to valine?
1. (9 Points) Almost a century ago, Franz Knoop studied
the metabolism of a series of ?-fatty acids by feeding them to dogs and
isolating the products in urine. As shown below, the products were either
derivatives of benzoic acid or phenyl acetic acid depending upon whether
the number of methyene groups was odd or even, respectively. From these
results he deduced the basic chemistry of the ?-oxidation pathway. From
the structures of products, hippuric or phenylaceturic acid, I want you
to make some deductions about the enzyme reaction that generates these
end products.
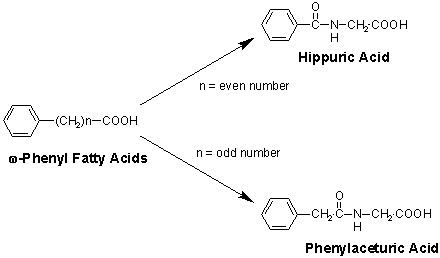
A. (3 Points) Name and draw the structure of the common
nitrogen-containing compound that has been added in the final metabolic
step.
B. (6 Points) Based on what you know about the beta-oxidation
pathway and the formation of certain bonds in biochemical systems, make
a reasonable proposal for the enzymatic reaction (substrates and products)
of the final metabolic step.
2. (10 Points) The pentose phosphate pathway is quite
versatile in the way it can respond to metabolic needs. For example, it
can produce pentose phosphates for nucleotide synthesis without involving
the oxidative phosphogluconate shunt as is depicted below for the formation
of 3 moles of ribose-5-P from two moles of F-6-P and one mole of GAP. In
principle, one could convert 5 moles of glucose to 6 moles of ribose this
way.
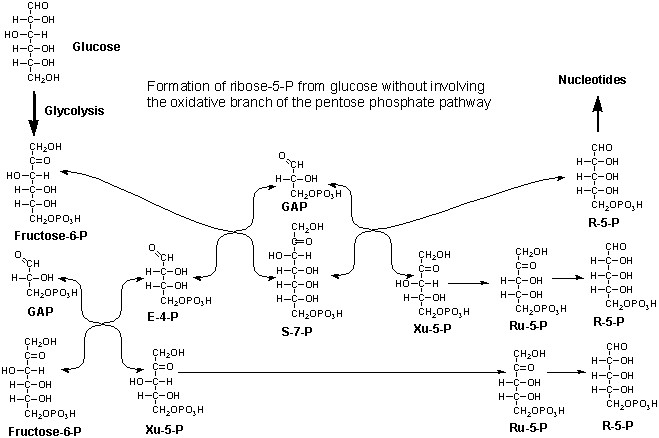
If one were to track a 14C label placed in the number
3 carbon of glucose through this pathway into ribose, what would be the
expected distribution of labeled carbon in the carbons of R-5-P? (Be careful)
Carbon 1 _____% Carbon 2 _____% Carbon 3 _____%
Carbon 4 _____% Carbon 5 _____%
3. (19 Points) Lysine, an essential amino acid for animals,
can be synthesized by bacteria, algae, fungi, and higher plants. Two completely
different biosynthetic pathways for lysine have evolved; one starting with
aspartate and the other with ?-ketoglutarate. The intermediates in the
latter pathway found in Neurospora crassa (a fungi) are shown below.
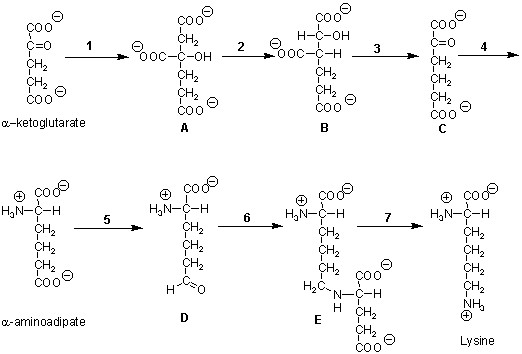
A. (2 Points) What would be a likely donor of the two carbon moiety added in Reaction 1?
B. (3 Points) Step 3 is an oxidative decarboxylation. What redox cofactor should this step use? Why?
C. (2 Points) Steps 1 through 3 in sequence are analogous to what other more familiar sequence of reactions? (2 Point Bonus - In what fundamental way does reaction 1 differ in the two sets of reactions?)
D. (2 Points) What cofactor might be expected for Step 4?
E. (2 Points) Step 5 is analogous to the formation of glyceraldehyde-3-P from 3-phosphoglycerate in the Calvin Cycle and gluconeogenesis. What cofactor requirement in addition to NADPH would one expect in Step 5?
F. (4 Points) One could conceive of a simple reaction converting Compound D to lysine directly. This obviously does not happen given the existence of Compound E. different redox coenzymes are used for these steps. Predict which is used in each case and provide an explanation.
G. (4 Points) A mutant lacking Step 4 activity might be
expected to accumulate Compound C. This mutant will grow in the presence
of DL-?-aminoadipate but no longer accumulates C. What might be a reasonable
explanation for this phenomenon?
1. (5 Points) One of the principles of metabolism is that
biochemical pathways are compartmentalized. To avoid futile cycles or diverting
common intermediates into the wrong pathway, pathways are active at different
times or in different cellular compartments or different tissues.
Another form of compartmentation was described in lecture whereby there
was neither temporal or physical separation. Conceptually describe conditions
that need to exist for two different redox systems to coexist in a cell
and not interfere with each other.
2. (5 Points) In Problem Set 1, you researched a particular
inborn error of metabolism before we had discussed any of the pathways
involved. What was the genetic disease you studied? Consider the various
principles of metabolism and apply at least two to the pathway affected
by the disease you studied.
3. (10 Points) Nearly a million children die each year from malaria, mostly in sub-Saharan Africa. Plasmodium falciparum, a protozoan parasite that causes malaria, has evolved resistance to quinine, chloroquine, and a variety of other anti-malarial drugs. Rational development of new and effective anti-malarial drugs requires knowledge of the parasite and its host, if one wants selectively to kill the parasite and spare the host. In general, parasites have simplified metabolism because they take advantage of what their hosts can offer. Within the human red blood cell, which lacks intracellular membranes, P. falciparum makes large amounts of membrane phospholipids for its own membranes and uses choline obtained from the blood, because it cannot synthesize it itself. A recent Science [295, 1311 (2002)] article describes a tight-binding inhibitor of choline uptake by Plasmodium that is considered by the authors to be a promising lead for anti-malarial drugs.
A. (5 Points) Based on the above and your knowledge, draw a conceptual diagram of choline metabolism in the malaria parasite in relation to the host red blood cell showing where the inhibitor interferes. (You need not provide detailed chemical structures.)
B. (5 Points) If you were hired to work on choline metabolism
as a possible anti-malarial target for drugs, what more would you like
to know? Why? Be concise because rambling answers will be ignored.