There are 17 pages to this examination. Put your name on each new page. A genetic code chart is on the last page. A separate set of pages contain the metabolic pathways we have discussed in class. If you feel any question is unclear or ambiguous, clearly explain your answer or interpretation.
Do not expose your answers to the scrutiny of your neighbors. Please fold under each page before you go on to the next.
Breakdown of the exam by sections:
I. Structures
34 Points
II. Short Answer
30 Points (+4 Bonus)
III. Multiple Choice
45 Points
IV. Problems
62 Points (+3 Bonus)
V. Short Essay
27 Points (+ 9 Bonus)
Total
200 Points
Possible Bonus
16 Points
Part I. Structures ( Points)
1. (8 Points) The structure of Acetyl Coenzyme A below contains a variety of functional groups and sub-components. Put a box around and label the following:
A. A thioester B. A phosphate ester C. An amide D. A glycosidic bond
E. Pyrimidine ring F. Imidazole ring G. A methylene group H. A carbonyl group
2. (10 Points) The following five cofactors are
involved in the glycine cleavage reaction as well as many others. Name
each and indicate the kind of reaction they normally help catalyze.
| Name:
Function: |
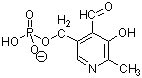 |
Name:
Function: |
|
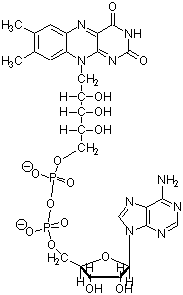 |
Name:
Function:
|
Name:
Function: |
|
| Name:
Function:
|
I. 3. (8 Points) During protein synthesis, tRNAs
go through a cycle in which they become aminoacylated at their 3' -CCA
end with their cognate amino acid, bind to the A-site on the ribosome,
undergo a transpeptidation reaction to form a peptidyl tRNA, translocate
to the P-site, transfer the peptidyl moiety to the next aminoacyl tRNA,
and then dissociate to repeat the cycle. Consider a tRNATyr
participating in normal protein synthesis and bearing a dipeptide. Draw
the complete structure of the expected dipeptide attached to the tRNATyr.
4. (8 Points) Draw the structure of the 3' -CA-dinucleotide at the end of tRNATyr and show where and how the dipeptide above is covalently attached to this end of the tRNA.
Part II. Short Answer (30 points, 1 point each)
The following 30 questions represent a random sample of vocabulary, definitions, terms, and concepts; fragments of information that you should have encountered this semester. By themselves, they are of little importance. However, knowing such bits of information is useful when put in the context of problems and new information you will encounter for the rest of your life.
_____________________________ 1. The -helix is an example of what level of protein structure?
_____________________________ 2. If [S] = Km, v = ____Vmax?
_____________________________ 3. An amino acid with no chiral centers.
_____________________________ 4. A catalyst does not change the _________ of a reaction.
_____________________________ 5. Name for the region of an enzyme surface involved in catalysis
_____________________________ 6. A plant glucose polymer linked by 1-4 glycosidic bonds.
_____________________________ 7. A plant glucose polymer linked by 1-4 glycosidic bonds.
_____________________________ 8. Physical property that distinguishes tRNA from rRNA.
_____________________________ 9. Chemical property that distinguishes tRNA from rRNA.
_____________________________ 10. Methotrexate mimics this cofactor.
_____________________________ 11. Uracil is a _____.
_____________________________ 12. Uridine is a _____.
_____________________________ 13. Uridylate is a _____.
_____________________________ 14. Name for the fatty acid designated C 18:0.
_____________________________ 15. Feature of the 3' end of most eukaryotic mRNAs.
_____________________________ 16. Organelle where oxidative phosphorylation occurs
_____________________________ 17. Organelle where glycoproteins become glycosylated.
_____________________________ 18. Enzyme that catalyzes transcription.
_____________________________ 19. Method for amplifying specific DNA or RNA sequences.
_____________________________ 20. Puromycin inhibits this process.
_____________________________ 21. An amino acid that disrupts -helices.
_____________________________ 22. Location in a eukaryotic cell where histones are found.
_____________________________ 23. Location in cells where lecithin is found.
_____________________________ 24. Protein substrate modified by diphtheria toxin.
_____________________________ 25. Vitamin precursor for FMN and FAD.
_____________________________ 26. Compound that accumulates in gout.
_____________________________ 27. Enzymes that use ATP are called ____.
_____________________________ 28. The "TATA Box" is a recognition sequence for ____.
_____________________________ 29. Thymine dimers form in the presence of _____.
_____________________________ 30. Metal ion associated
with chlorophyll.
Nobel Prize Winner Trivia (4 Bonus Points).
_____________________________ 31. Two who unraveled DNA; the second a pain in the neck.
_____________________________ 32. One with two cycles named for him, but not a bicycle.
_____________________________ 33. A "musical" sequencer
with two,
one ditty with dideoxy in the land of Di.
_____________________________ 34. Vitamin C came after his two.
For questions 1 through 4, there are five items listed. Four of them have something in common which the fifth does not. Your objective is to determine which of the five is least related to the other four and state briefly what links the four that does not include the fifth. No credit for answers without reasons.
____ 1. A. Oxaloacetate
B. Aspartate C. Succinate
D. Citrate E. Glutamate
Shared feature __________________________________________________________________
____ 2. A. Glucose 6-phosphate B. 3-Phosphoglycerate C. Dihydroxyacetone phosphate
D. Fructose 6-phosphate E. Ribose 5-phosphate
Shared feature __________________________________________________________________
_____3. A. Glucose
B. Sucrose C. Mannose
D. Galactose E. Fructose
Shared feature __________________________________________________________________
_____ 4. A. Lysine
B. Ornithine C. Arginine
D. Alanine
E. Valine
Shared feature __________________________________________________________________
____ 5. In 1906, Harden and Young observed that
inorganic phosphate (Pi) is an essential requirement for fermentation
of glucose in a cell-free yeast extract. If 32P were available
then, they could have used it show that phosphate becomes covalently attached
to intermediates in glycolysis and other compounds. Imagine the fate of
a phosphate ion placed into a fermenting cell-free yeast extract. Which
of the following would be the first organic phosphate formed?
A. ATP B. Glyceraldehyde 3-phosphate C. Phosphoenolpyruvate
D. Glucose 6-phosphate
E. 1,3-Bisphosphoglycerate.
Time out for reflection:
"Man's interest in alcoholic fermentation
needs no explanation in itself. Understandably, stimulus for scientific
discovery has come from aims less exalted than disinterested pursuit of
knowledge. Many of the alchemists who prepared the way for modern chemistry
were motivated by the greed for gold. Biochemistry owes as much to man's
thirst for alcohol as chemistry does for his appetite for money. Early
impetus came for the quest for liquor in one case , for lucre in the other."
F.
R. Jevons in The Biochemical Approach to Life (1964)
____ 6. Which of the following conforms to the patterns of nature expected in biochemistry?
A. Triglyceride
containing a fatty acid symbolized by C17:1.
B. A nucleic
acid with bases linked by -N-glycosidic bonds.
C. A protein
containing only L--amino acids.
D. A nucleic
acid containing 2',5' phosphodiester bonds.
E. D and L
isomers being equally good substrates for an enzyme.
____ 7. Consider the metabolic differences between birds and mammals. Which of the following enzymes would you expect to be significantly more concentrated in human liver than in chicken liver?
A. IMP dehydrogenase B. Lactate dehydrogenase C. Arginase
D. Succinate dehydrogenase
E. DNA polymerase
____ 8. Which of the
enzymes above would be more concentrated in chicken liver than human liver?
____ 9. Pick the false statement:
A. Semiconservative replication is characteristic of DNA synthesis in humans and bacteria.
B. The base composition of DNA is unaffected by age in an organism.
C. The base composition of mRNA conforms to Chargaff's rules.
D. If one intron of many were not spliced out in forming an mRNA,
the encoded protein would likely be shorter than normal.
E. In B-DNA, the base pairs are perpendicular to the helical axis.
____ 10. & ____ 11. In the absence of the vitamin niacin, we can synthesize the nicotinamide ring of NAD from the essential amino acid tryptophan. (By the way prevet students, cats can't do this.) An intermediate in this pathway is quinolinic acid (shown below). All of the carbon atoms and the nitrogen atom of quinolinic acid come directly from tryptophan. If the indicated adjacent carbon atoms in tryptophan were labeled with 13C, their nuclear spins would interact and be apparent in a 13C NMR spectrum. However, the 13C NMR spectrum of quinolinic acid derived from this doubly labeled tryptophan would not have coupled nuclear spins even though neither carbon is lost. Based on this information, which positions would have 13C?
____ 12. Among the dogmas of biochemistry that are generally accepted are that:
A. All biological membranes contain cholesterol.
B. Amino acid sequence determines tertiary protein structure.
C. DNA is a template for RNA synthesis, but never the reverse.
D. The genetic code is universal.
E. All enzymes are proteins.
____ 13. The hypothetical
disaccharide, verbose, extracted from discarded term papers at the Writing
Center contains glucose linked to sedoheptulose as shown below. The glycosidic
bond in verbose is:
|
|
A. , 1-4
B. , 1-5 C. , 2-5 D. , 1-3 E. , 1-4 |
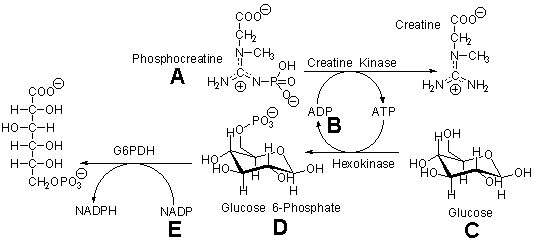
____ 14. Tissue damage resulting from a heart attack often results in the release of intracellular enzymes into the blood stream where they are not normally found. The amount of creatine kinase in the blood following a heart attack is used as an indicator of the extent of tissue damage. Automatic clinical analyzers measure the amount of creatine kinase in a blood sample by a coupled assay which measures the rate of NADPH production by glucose 6-phosphate dehydrogenase (G6PDH). The reactions used in the assay are shown below. Which of the lettered components should not be present initially in the assay mixture?
____ 15. Bacitracin is a polypeptide antibiotic produced by Bacillus licheniformis. Its amino acid sequence prior to modification is:
Ile-Cys-Leu-Glu-Ile-Lys-Orn-Ile-Phe-His-Asp-Asn
From this sequence, one might deduce that bacitracin,
A. Is not synthesized on a ribosome.
B. Does not contain sulfur.
C. Is negatively charged at pH 7.0.
D. Contains 15 nitrogen atoms.
E. Contains a hydroxyl group.
The scope of biochemistry far
exceeds the amount of material that can be covered in a one semester course.
One of my major goals for CHEM-527 was to help you gain the basic knowledge
and skills that would enable you to make sense of new information and provide
you with the basis for continued learning. Thus, the following section
contains material on amino acid metabolism that you may have encountered
in your text, but which I have not discussed in class and do not expect
you to know. However, I do expect you to understand the vocabulary and
apply basic principles to answer the questions.
IV.1. The following diagram outlines the biosynthetic pathway for threonine and relates it to the biosynthesis of other amino acids derived from aspartic acid.
A. (10 Points) Two intermediates (A & B) on the pathway to threonine are omitted along with coenzymes associated with reactions C, D, E, & F. Based on similarity to reactions portrayed on the metabolic handout sheets, predict the structures of the missing intermediates and identify the cofactors.
Coenzymes for Reactions:
C =
D =
E =
F =
Compound A Compound B
B. (4 Points) Threonine
inhibits three of the five enzymes in its biosynthesis from aspartate.
Which three would they be? Does this make good metabolic sense? What makes
these enzymes different from the other two?
IV. 1. C. (4 Points) Threonine, an amino acid with two chiral centers, is the precursor of isoleucine, the only other common amino acid with two chiral centers, as is shown below. Put a circle around the carbon atoms in isoleucine that are derived from pyruvate.
D. (4 Points) Interestingly,
each of the enzymes above has dual substrate specificity such that a methyl
group can replace the ethyl group. Thus, in contrast to isoleucine, all
of the carbon atoms of valine synthesized by this pathway come from pyruvate.
Acetolactate synthase, the TPP-dependent enzyme, is the target for the
potent sulfonylurea herbicides, Oust® and Glean®, manufactured
by Dupont [Trends in Biotech.2(6), 158-161 (1984)]. In cells inhibited
by these compounds, -ketobutyrate accumulates but pyruvate does not. How
do you explain this?
E. (4 Points) While
Oust® and Glean®, with Ki values in the nM range, are
toxic to plants at a few grams per hectare, they have low toxicity to animals.
What is a reasonable explanation for this large difference in toxicity?
VI. 1. F. (8 Points)
In addition to transamination to yield valine, -ketovalerate also is a
precursor to leucine. Examine the chemical relationship between valine
and leucine and the corresponding -ketoacids that get transaminated to
form each. Now consider the metabolic steps that relate another more familiar
pair of -ketoacids bearing the same relationship. Using these hints, write
out the sequence of biosynthetic reactions that convert -ketovalerate to
leucine.
IV. 2. A bacterial cell must be sensitive to nutrients in its environment so that it does not waste energy synthesizing those compounds when they are available. For example, E. coli can synthesize all of the amino acids found in proteins. If one or more of these amino acids becomes available, the biosynthetic pathways for them are shut down by end product inhibition and the synthesis of the enzymes of the pathways is repressed. As a result of DNA sequence and RNA sequence analysis combined with the physiologic response of various regulatory mutants, the fine control of the repression process is fairly well understood.
This question will focus on the exquisite fine control known as attenuation. The following are some of the features of the attenuation mechanism quoted or paraphrased from Keller & Calvo [PNAS, 76, 6186-90 (1979)].i. Most transcription initiated at the relevant promotor terminates before the structural genes of the operon are reached, resulting in the synthesis of a leader RNA of about 150 nucleotides.
ii. The site at which termination occurs ("attenuator") is similar to previously identified transcription termination sites. It is a palindromic G-C rich region followed by a series of adenosines on the coding strand. The corresponding region of the leader RNA, which has a potential stem and loop structure followed by a series of uridines, is called the "terminator."
iii. Each of the known leader RNAs contains a second potential stem-and-loop structure proximal to the terminator and overlapping with it in such a way that pairing with one region precludes pairing with the other.
iv. Within each leader RNA, translational start and stop signals are positioned so that a peptide of 14-28 amino acids might be synthesized.v. Each leader peptide contains in high frequency the amino acid corresponding to the particular operon.
vi. The derepression of the operon requires the transcriptional read-through of the attenuator. This occurs only if a ribosome initiates the synthesis of the leader peptide and is retarded in its progress by lower than normal amounts of a specific amino acyl tRNA. This favors a different conformation of the leader RNA and signals transcriptional read through.
The sequence on the next
page corresponds to the non-coding strand of DNA corresponding to
the control region of a particular amino acid operon [PNAS 76(4),1706-1710
(1979)]. Like mRNA, it is complementary to the template or coding strand.
A genetic code sheet is provided for you assistance in some of the questions
that follow.
5'...ACAGATAAAAATTACAGAGTACACAACATCCATGAAACGCATTAGCACCACC
ATTACCACCACCATCACCATTACCACAGGTAACGGTGCGGGCTGACGCGTACA
GGAAACACAGAAAAAAGCCCGCACCTGACAGTGCGGGCTTTTTTTTTCGACCA
AAGGTAACGAGGTAACAACCATGCGAGTGTTGAAGTTCGGCGGTACATCA...3'
VI. 2. A. (2 Point) Underline the transcriptional termination region.
B. (2 Points) This DNA sequence corresponds to that of the relevant leader RNA and mRNA. Circle the initiation and termination codons of the leader peptide.C. (2 Point) Put a box around the initiation codon for the first structural gene.
D. (5 Points) Write the predicted amino acid sequence of the leader peptide above the appropriate codons. (3 point bonus if you can use the correct one letter representations for the amino acids.)
E. (3 Points) This sequence is derived from the control region of the operon for what amino acid?
F. (3 Points) Identify the palindromic region corresponding to the terminator with opposing arrows drawn above the sequences.
G. (3 Points) Identify the second palindromic region which overlaps the terminator with opposing arrows drawn under the sequences.
H. (6 Points) Depict in a general way the alternative base-paired structures possible for the leader RNA corresponding to the above sequence of DNA. Which conformation would be favored by low levels of the relevant amino acid?
I. (4 Points) Attenuation is an extremely elegant and finely tuned mechanism for controlling the express ion of amino acid operons. One must consider that even higher order regulation is involved such as that between operons. Consider the metabolism of the amino acid you have identified in Part E and the sequence of the leader peptide from Part D. What other amino acid seems to be important in controlling the expression of this operon? Does this make metabolic sense? If so, explain.
V. Essay Questions (9 Points each) Answer 3 out of 4. Answer all 4 for bonus points.
This part of this examination attempts to assess your learning and ability to communicate clearly. Writing reflects how you think. Among the "right answers" I will read, some will be better than others because they show a greater depth of understanding, provide a more logical structure, and use words with precision. Better quality answers will receive higher marks. Therefore, organize your thoughts before you write.
A. A man fed a low
protein-high carbohydrate diet excreted 6.2 grams of urea per day. After
three days without food, the same man excreted 26.2 grams of urea per day.
Explain these data.
B. ATP has been described
as nature's dehydrating agent. Using examples, show what this means.
V. 3. C. Describe
conceptually how one determines the nucleotide sequence of a piece of DNA.
What aspects are the critical for the procedure to work?
D. Your textbook does
not include tRNAs as coenzymes. What are coenzymes? Would you consider
tRNAs as coenzymes? Why or why not?
Genetic Code Chart
| UUU | PHE
|
UCU | SER | UAU |
TYR |
UGU |
CYS |
| UUC | UCC | UAC | UGC | ||||
| UUA |
LEU |
UCA | UAA | End | UGA | End | |
| UUG | UCG | UAG | UGG | TRP | |||
| CUU | CCU | PRO | CAU | HIS | CGU | ARG | |
| CUC | CCC | CAC | CGC | ||||
| CUA | CCA | CAA | GLN | CGA | |||
| CUG | CCG | CAG | CGG | ||||
| AUU | ILE | ACU | THR | AAU | ASN | AGU |
SER |
| AUC | ACC | AAC | AGC | ||||
| AUA | ACA | AAA |
LYS |
AGA |
ARG |
||
| AUG | MET | ACG | AAG | AGG | |||
| GUU | VAL | GCU | ALA | GAU |
ASP |
GGU | GLY |
| GUC | GCC | GAC | GGC | ||||
| GUA | GCA | GAA |
GLU |
GGA | |||
| GUG | GCG | GAG | GGG |