CHEM-342
Introduction to Biochemistry
Name ______________________________
Midterm Examination - Individual
Part
Friday, 29 March 2002
H. B. White - Instructor
Important - Please read this before you turn the page.
-
Write your name on each page
-
This part of the midterm examination is worth 80 points distributed
as follows:
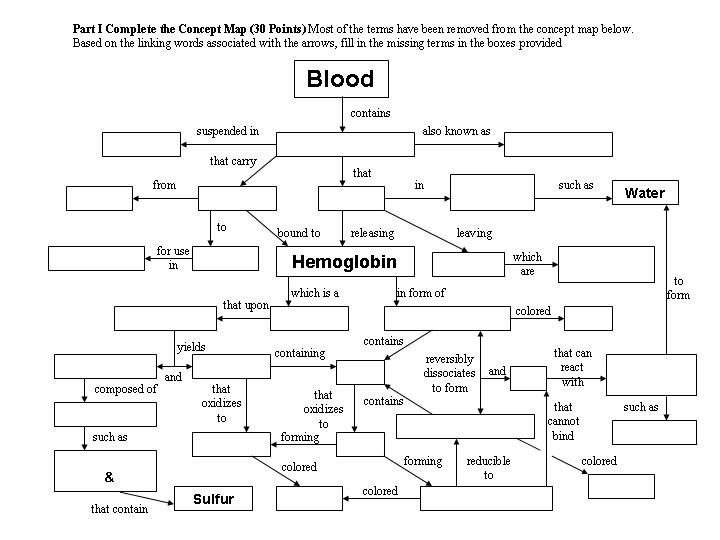
I. (30 points)
Fill in the Blank Concept Map
II. (12 points) 3
Short Answer Questions.
III. (19 points) Problem
and Short Essay Questions.
IV. (14 points) Essay
V. (5 points)
Bonus Question
-
You may refer to your notes, course reader, handouts, or
graded homework assignments. Textbooks or reference books cannot be used.
-
This examination will assess your learning, problem-solving
skills, and ability to communicate clearly. It is intended to be
challenging even to the best students in the class.
-
Writing reflects how you think. Among the “right answers”
I will read for the following questions, some will be better than others
because they show greater depth of understanding, avoid extraneous or inaccurate
information, provide a more logical structure, use appropriate examples,
and chose words with precision. Better quality answers will receive
higher marks. Therefore organize your thoughts before you write.
-
Strive to write not that you may be understood, but rather
that you cannot possibly be misunderstood. Stream of consciousness
answers are rarely well organized or clearly presented.
-
Have a relaxing and safe Spring Break.
Exam Statistics
Number of students
18 Ave.
Range
(out of 80 points)
Number of groups
5 Ave.
20.3
Range 17 - 23 (out of 30 points)
Class Totals
Ave.
Range
(out of 100 points)
Part II Three Short Answer Questions. (4 Points each)
1. Explain clearly why Zinoffsky's repeatedly crystallized
horse hemoglobin until the elemental analysis of the mother liquor matched
that of the crystals? (Here and elsewhere on this exam, pictures to augment
words are especially welcome.)
2. Peters used ferricyanide to oxidize hemoglobin to methemoglobin
and release the bound oxygen, which he measured as a gas. But, oxygen is
soluble in water and was released from hemoglobin in solution. How is it
possible to quantitatively measure oxygen volumetrically as a gas when
some of it is dissolved in solution?
3. Venous blood contains deoxyhemoglobin. Where did the
oxygen go? How is this similar to Stokes' experiment?
Part III Problems and Short Essays
1. (9 Points)
When Professor Essigsaure first tried to repeat the experiment
in Section 11 of Stokes' article he was in a hurry (as usual) and tried
to cut corners. As described, he added an equal volume of ether to his
dilute solution of blood. Then he added the glacial acetic acid to the
mixture until the color turned brown and mixed it gently until the hematin
extracted into the ether phase. After washing the ether layer once, his
patience began to wear. Rather than washing the ether layer until
the hematin precipitated at the interface, he went directly to the last
step and added some bicarbonate solution expecting to have the hematin
transfer into the aqueous layer. Fortunately, he was wearing lab goggles
and protective gloves because the solution erupted in froth and overflowed
the container spilling over his hands.
A. (3 Points) What is the purpose of washing
the ether layer? Draw a picture or equation of what is happening.
B. (3 Points) Why did the solution erupt when the bicarbonate
was added? Write an equation to help explain your answer.
C. (3 Points) Would the solution have erupted in froth
had Prof. Essigsaure used a dilute ammonia solution rather than bicarbonate?
Explain.
2. (10 Points)
Peters (1912) used an earlier version of what came to
be known as the Warburg Constant Volume Manometer Apparatus shown below.
The apparatus shows the appearance at the beginning of an experiment. Assume
that an oxygen-generating reaction has occurred in the reaction vessel
to produce some volume of gas.
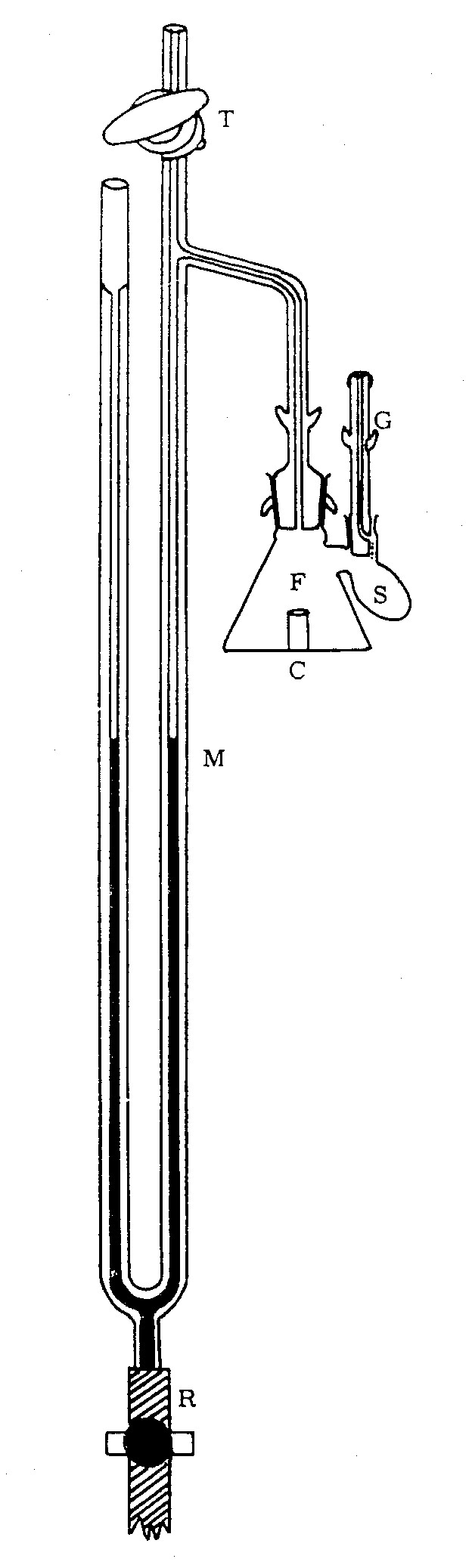 |
A. (5 Points) Show how the clove oil would be displaced
and how its level would be adjusted before a reading would be made?
Image copied from Umbreit, Burris, and Stauffer,
ManometricTechniques, 4th ed. 1964 |
B. (5 Points) What properties does clove oil have
that makes it particularly suitable as the displaced fluid in the manometer?
Part IV Essay (14 Points)
There are three quotations below reflecting various views
of the nature of science. Select one to defend or refute using examples
and evidence taken from the articles you have read so far this semester
Stokes (1864), Zinoffsky (1886), Peters (1912), Conant (1923), and Svedberg
& Fåhraeus (1926). Use the back of this page to organize your
answer, if you need more room.
Progress in science depends on new techniques, new
discoveries, and new ideas, probably in that order.
Sydney Brenner
…scientists love to do experiments that show their
colleagues to be wrong. By this adversarial process, science gradually
reveals the way nature works. The notion that published science must be
free of errors, and that error itself indicates sloppy thinking or fraudulent
intent, is misguided.
Robert Pollack
It is all too easy, for scientists and non scientists
alike, to assume science produces truth. What it generally produces however,
are models: deliberate in their assumptions, limited in their scope, provisional
in their conclusions, and extraordinarily useful for certain purposes,
even perhaps certain causes. But they are neither perfect, nor permanent.
Frank H. T. Rhodes
Part V Bonus (5 Points)
The arterial blood of a typical college student contains
about 16g of Oxyhemoglobin per 100ml. Estimate how many ml of oxygen per
100ml of blood does that represent at standard temperature and pressure?
(Note: Estimation does not require a calculator. Any answer within a factor
of 2 is correct. Show work for credit.)
Return to Hal
White's Home Page, CHEM-342
Home Page, or Departmental Home
Page.
Last updated: 29 March
2002 by Hal White
Copyright 2002, Department
of Chemistry and Biochemistry, University of Delaware


