CHEM-342 Introduction to
Biochemistry
Name ________________________
Final Examination - Individual
Part
Tuesday, 21 May 2002
7:00 - 8:45 PM
H. B. White - Instructor
Important - Please read this before you turn the page.
There are 7
pages to this part of the examination. Please write your name on each page.
Part I (75
points) This individual part of the examination, includes 9 problems and
short essay
questions.
Part II (25 points) The group part of the examination,
has one problem with multiple parts.
There is a 5 point bonus question in each part.
If you complete Part I early, you may leave the room for
a break and return at or before 8:45 PM when Part II begins.
You may refer to your notes, course reader, handouts,
or graded homework assignments. Textbooks and the course book resources
can be used for Part II only.
This examination will assess your learning, problem-solving
skills, and ability to communicate clearly. It is intended to be
challenging even to the best students in the class. Writing reflects
how you think. Among the “right answers” I will read for the following
questions, some will be better than others because they show greater depth
of understanding, avoid extraneous or inaccurate information, provide a
more logical structure, use appropriate examples, and choose words with
precision. Better quality answers will receive higher marks.
Therefore organize your thoughts before you write. Strive to write not
that you may be understood, but rather that you cannot possibly be misunderstood.
Stream of consciousness answers are rarely well organized or clearly presented.
Attempt to draw a picture or diagram as part of your answer
to every question.
Graded examinations may be picked up Friday morning, 24
May.
Have a relaxing and safe Summer.
Examination Stats: Range - 26
to 72 out of 80, Average - 72.3,
Number - 18
1. 1. (5 points) What is the significance
of the increased number of nucleated red blood cells (reticulocytes) that
Herrick saw in his patient with sickle-cell disease, a hemolytic anemia?
Your answer needs to make a logical connection between the phenomenon and
the disease.
2. (5 points) Linus Pauling advocated consumption of megadoses
of Vitamin C to combat the common cold and other ailments. He took 10 g/day
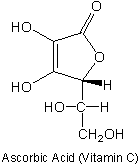
and lived to 93. Ascorbic acid (aka Vitamin C) has a pKa of 4.17. Put a
circle around the dissociable proton. Explain your choice.
3. (5 points) Ingram used 2,4-dinitrofluorobenzene for
determination of the amino terminal amino acid in a peptide. Later he used
phenylisothiocyanate (Edman's Reagent), which is now preferred. What
are the merits of Edman's reagent compared to DNFB.
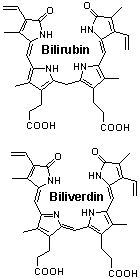
4. (5 points) Bilirubin and biliverdin,
drawn below, are breakdown products of heme. They provide the array of
colors seen in a bruise. Structurally, they differ by the reduction of
a double bond. Their two carboxyl groups appear virtually identical. However,
the pKa values for them are different. In one case the values are 4.2 and
4.9 while in the other they are 3.9 and 5.3 [J. Biol. Chem. 271, 2397 (1996)].
Predict which set of values goes with which compound. State the basis for
your prediction.
5. (5 points) The function of hemoglobin is tightly coupled
to its structure. The effects of mutation affecting a single amino acid
can range from benign to lethal as is seen in the variety of hemoglobinopathies
studied by you and other students in the class. List 5 conceptually different
ways a structural change in hemoglobin can affect its function. Start with
the variant you studied.
A.
B.
C.
D.
E.
6. Fig 1 is from Ingram's first article.
It monitors the time course for the digestion of HbA and HbS by trypsin
at pH 8. NaOH is added to maintain the pH.
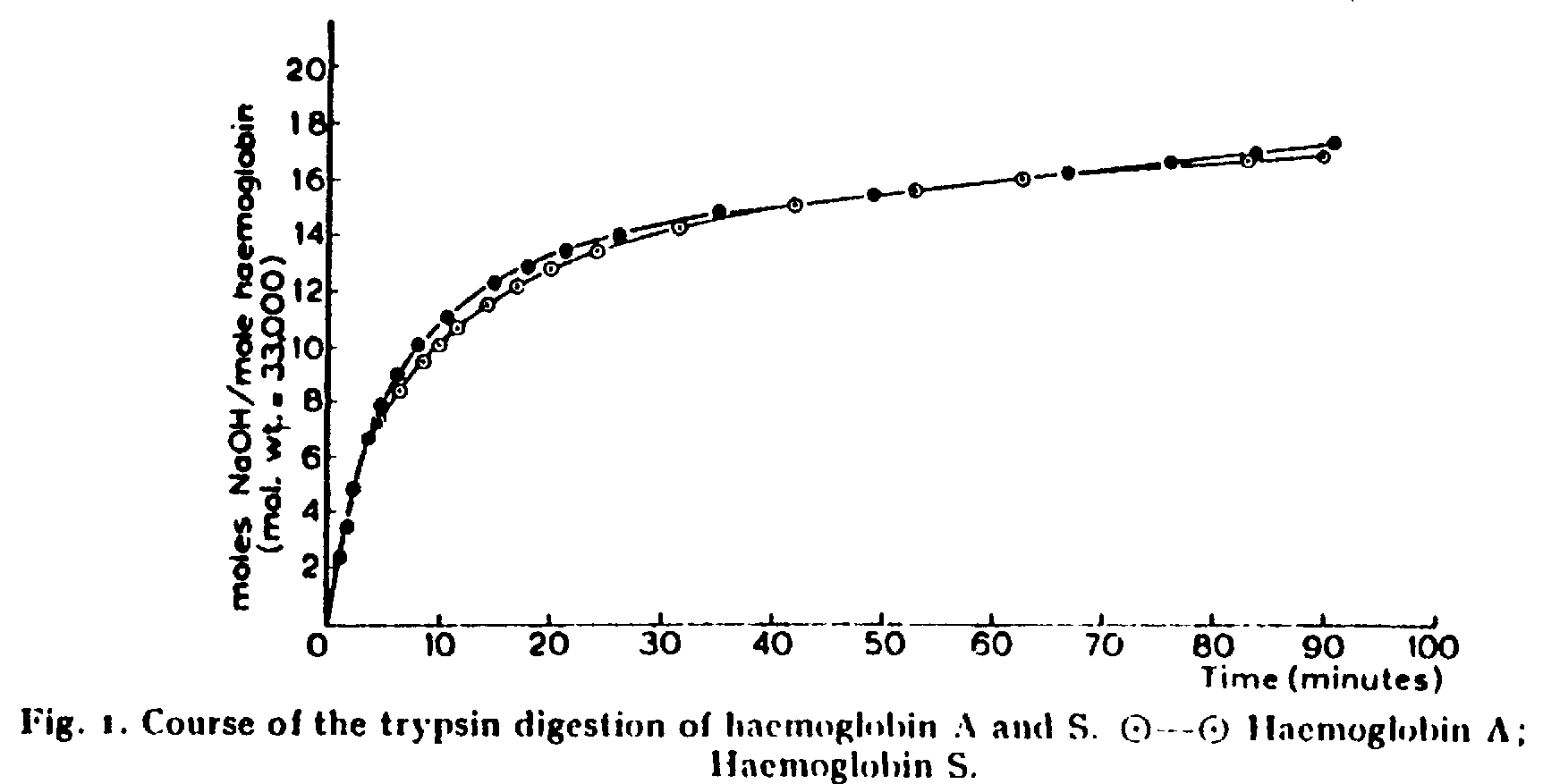
A. (5 points) Chemically describe in words and structures
the reaction catalyzed by trypsin.
B. (5 points) Explain how there is a net production of
protons (H+) in this reaction
C. (5 Points) What would the curve look like (sketch it
in above) if the reaction in Figure 1 were carried out at pH 7.0 or 10,
rather than 8.0? Explain your answers.
D. (5 points) How does the number of equivalents of hydroxide
compare to the number of peptide bonds hydrolysed by trypsin?
7. (10 points) Professor Essigsaure has discovered
an electrophoretic variant of human hemoglobin that he wants to call HbGore
Hall. On electrophoresis at pH 8, its mobility suggests that it has two
more negative charges than HbA. Before he can publish anything about it,
he needs to show that it hasn't been described previously. First, he wants
to determine whether the mutation affects the ? or ? globin subunit. One
morning, as he was taking a shower, he had a Eureka! moment and almost
forgot to get dressed. Skipping breakfast, he ran most of the mile from
his home to his lab. From the freezer, he took a small sample of his carefully
purified HbGore Hall, thawed it, then incubated it with an equal amount
of HbS, and ran the mixture on electrophoresis. By noon jubilant Prof.
Essigsaure proclaimed that the mutation in HbGore Hall affected the ?-chain.
What did the electrophoretic pattern look like for him to make his conclusion?
You must draw a picture to receive credit. [Based on Itano & Robinson,
Nature 184, 1468 (1959)]
Part III - Short Essays
8. (10 points) While we know of hundreds of human hemoglobin
variants generated by single amino acid replacement, this is a small portion
of the number possible. Consider all the possible single amino acid replacements
that might occur. How large is that number? Make an estimate. Was Pauling
lucky that HbS had a charge difference? Explain.
9. (10 points) The fact that HbS confers resistance
to malaria appears in textbooks everywhere. It represents a classic demonstration
of natural selection operating on the human population and provides an
example of a heterozygote being fitter than either homozygote where malaria
occurs. When students read about the experiments reported by Allison (1954)
to test the hypothesis, they often are shocked. What did Allison do to
test the hypothesis? What about his experiment is shocking? Evaluate student
reaction in the context of: ethical standards have changed, the risks of
doing these experiments have changed, the details of the experiment are
not understood.
Bonus Question
10. (5 points) An adult human body contains about 2 grams
of iron of which about 70% is associated with hemoglobin. Estimate to within
an order of magnitude the number of hemoglobin molecules in your body.
(i. e. your answer needs only to be expressed as a power of 10.)
Return to Hal
White's Home Page, Course
Home Page, or Departmental Home
Page.
Posted: 23 May 2002 by
Hal
White
Copyright 2002, Harold B.
White, Department of Chemistry and Biochemistry, University of Delaware


