CHEM-342 Introduction to
Biochemistry
Name ________________________
Final Examination - Individual
Part
Friday, 25 May 2001
3:30 - 5:15 PM
H. B. White - Instructor
Important - Please read this before you turn the page.
-
There are 7 pages to this part of the examination. Please
write your name on each page.
-
Part I (75 points) This individual part of the examination,
includes 10 problems and short essay questions.
Part II (25 points) The group part of the examination,
has 2 problems, one of which has multiple parts.
There is a 5 point bonus question in each part.
-
If you complete Part I early, you may leave the room for
a break and return at or before 5:15 PM when Part II begins.
-
You may refer to your notes, course reader, handouts, or
graded homework assignments. Textbooks and the course book resources can
be used for Part II only.
-
This examination will assess your learning, problem-solving
skills, and ability to communicate clearly. It is intended to be challenging
even to the best students in the class. Writing reflects how you think.
Among the
Aright answers@
I will read for the following questions, some will be better than others
because they show greater depth of understanding, avoid extraneous or inaccurate
information, provide a more logical structure, use appropriate examples,
and choose words with precision. Better quality answers will receive higher
marks. Therefore organize your thoughts before you write. Strive to write
not that you may be understood, but rather that you cannot possibly be
misunderstood. Stream of consciousness answers are rarely well organized
or clearly presented
-
Graded examinations may be picked up Wednesday morning, 30
May.
-
Have a relaxing and safe Summer.
1. (4 points) What research seminar
did you attend? What did you learn?
2. (5 points) What is the causal relationship
connecting jaundice to anemia among people with sickle cell anemia?
3. (6 points) Allison (1954) notes that
newborn infants, regardless of their sickle cell genotype, appear to be
resistant to malaria. He correlates susceptibility with the disappearance
of red blood cells containing fetal hemoglobin in the months after birth
(Figure on p 293). For a child homozygous for the sickle cell gene, would
the appearance symptoms of anemia precede, parallel, or lag behind the
susceptibility to malaria in children homozygous for the normal gene for
hemoglobin? Explain your answer and draw a graph of your prediction.
4. (15 points) Advances in science
often follow the development of new techniques and methodology that can
be broadly applied. This semester you have encountered the spectroscope,
redox titrations, electrochemical titrations, equilibrium ultracentrifugation,
isotopic tracers, Tiselius electrophoresis, paper electrophoresis, paper
chromatography, and Edman degradation among others. Select one
of these techniques or methods. Describe it and the principle(s) on which
it is based. Include examples and diagrams, mathematical equations, and/or
chemical equations as necessary.
5. (5 points) Bonus Question. On the back
of this page, derive the Henderson-Hasselbalch Equation
6. The diagram below represents the
titration of riboflavin with dithonite as it was done in class on 12 February.

A. (5 points) On the above graph, sketch in another
graph which would show the expected changes in dissolved oxygen concentration
which starts at "X" on the graph.
B. (5 points) Explain, what is happening with oxygen
in each of the regions indicated on the graph.
7. Figure 1 from the Shemin and Rittenberg
(1946) article is reproduced twice below. Please sketch in the curves that
you would expect if the conditions of the experiments were changed in the
indicated ways. Your answers will be evaluated on their qualitative merits
and whether the depiction corresponds to the written justification you
provide.
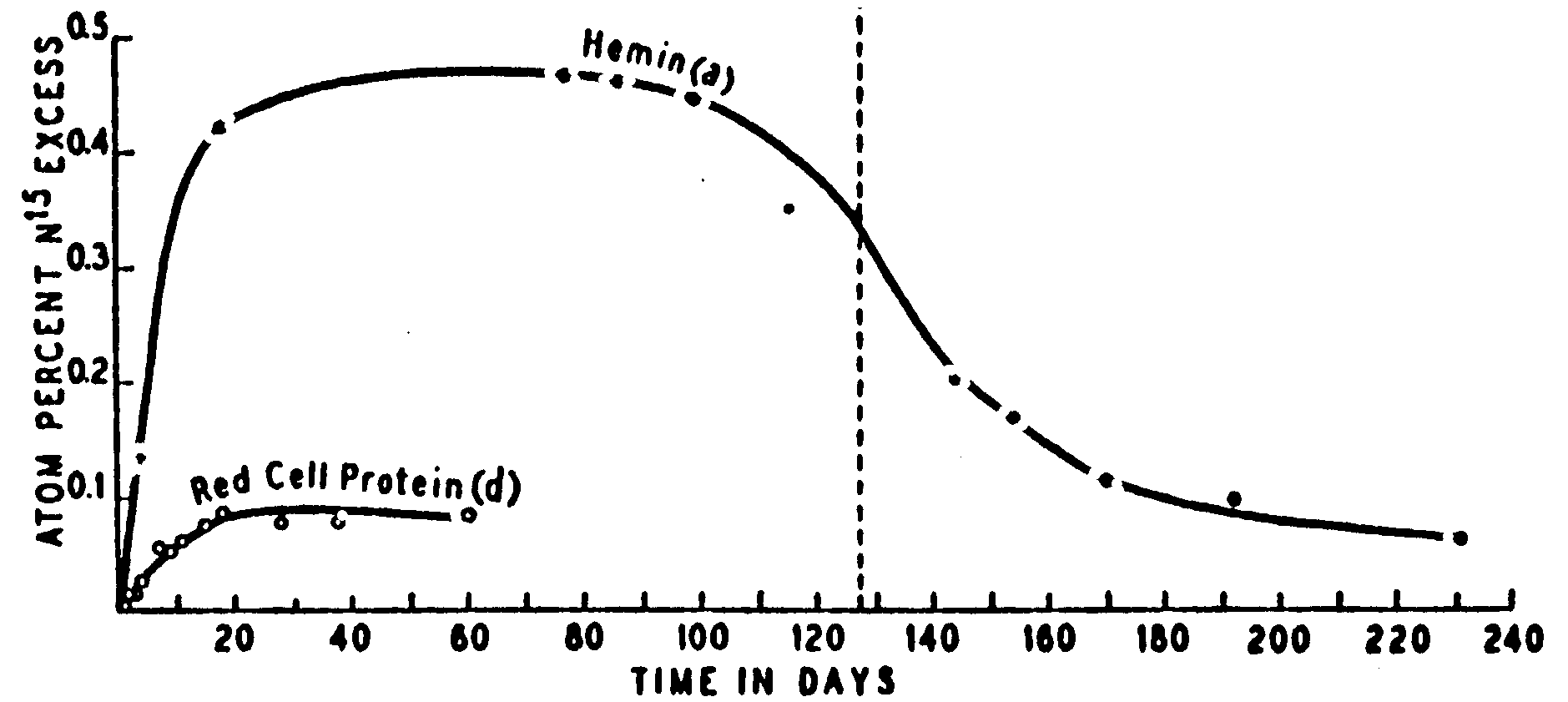
A. (6 points) What would the
hemin and red cell protein data look like if Shemin had a hemolytic anemia
like sickle cell anemia?
Explanation.

B. (6 points) What would the hemin
and red cell protein data look like if Shemin had ingested any other 15N
labeled amino acid than glycine?
Explanation.
8. On the next page, there is a representation
of the amino acid sequences of the alpha and beta globin chains of rabbit
hemoglobin.
A. (2 points) Put a circle
around every sulfur-containing amino acid residue in the rabbit hemoglobin
chains.
B. (5 points) If Zinoffsky had used
rabbit hemoglobin instead of horse hemoglobin for his determination of
the iron and sulfur content of hemoglobin, what stoichiometry of Fe to
S would he have found?
Explain your answer.
9. (2 points) Use small arrows to identify
the position of peptide bonds in the rabbit hemoglobin amino acid sequence
(next page) that would be hydrolyzed by trypsin.
A. (2 points) How long in
amino acid residues is the largest peptide you would expect in a complete
trypsin digest of rabbit hemoglobin?
B. (5 points) Because the alpha and
beta hemoglobin chains arose as a consequence of gene duplication, they
retain similarities to the ancestral amino acid sequence despite several
hundred million years of independent evolution. Would this similarity cause
any problems in distinguishing tryptic peptides derived from the rabbit
alpha chain with those from the rabbit beta chain?
10. Assume that, like Shemin, you wanted
to study hemoglobin synthesis but, because you wanted to use a radioactive
amino acid precursor, you decided on an animal model (rabbit) rather than
self-experimentation. Furthermore, for reasons of subsequent analysis,
you wanted to select an amino acid precursor that would label as many different
tryptic peptides as possible.
A. (3 points) Ignoring
expense and availability, which amino acid would be your choice?
B. (4 points) What
is the basis of your selection?
Rabbit Hemoglobin alpha chain
MVLSPADKTNIKTAWEKIGSHGGEYGAEAVERMFLGFPTTKTYFPHF
DFTHGSEQIKAHGKKVSEALTKAVGHLDDLPGALSTLSDLHAHKLRV
DPVNFKLLSHCLLVTLANHHPSEFTPAVHASLDKFLANVSTVLTSKYR
Rabbit Hemoglobin beta chain
VHLSSEEKSAVTALWGKVNVEEVGGEALGRLLVVYPWTQRFFESFGDL
SSANAVMNNPKVKAHGKKVLAAFSEGLSHLDNLKGTFAKLSELHCDKL
HVDPENFRLLGNVLVIVLSHHFGKEFTPQVQAAYQKVVAGVANALAHKYH
The single-letter abbreviations
for the 20 amino acids are:
A = Alanine
I = Isoleucine R = Arginine
C = Cysteine
K = Lysine
S = Serine
D = Aspartic Acid
L = Leucine T = Threonine
E = Glutamic Acid
M = Methionine V = Valine
F = Phenylalanine
N = Asparagine W = Tryptophan
G = Glycine
P = Proline Y = Tyrosine
H = Histidine
Q = Glutamine
Return to Hal
White's Home Page, Course
Home Page, or Departmental Home
Page.
Posted: 14 June 2001 by
Hal
White
Copyright 2001, Harold B.
White, Department of Chemistry and Biochemistry, University of Delaware

