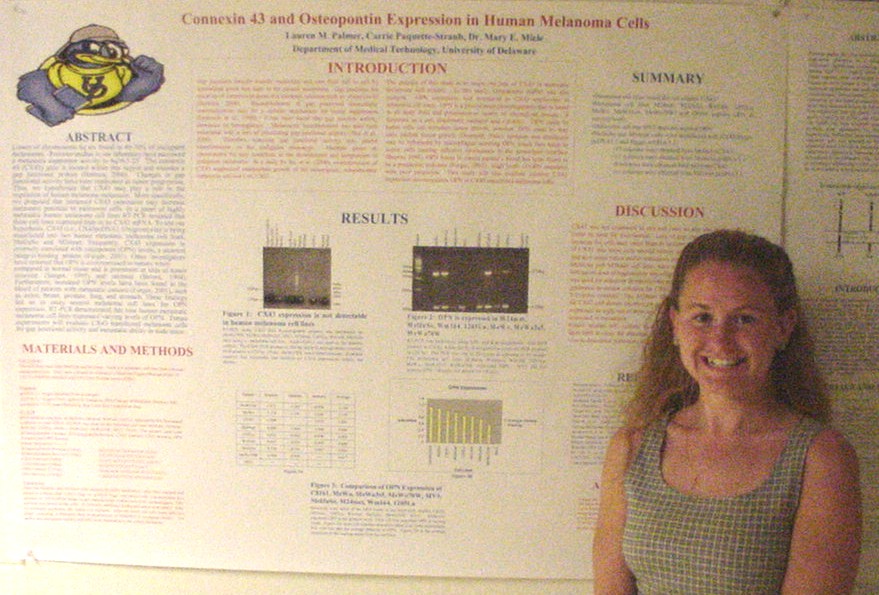
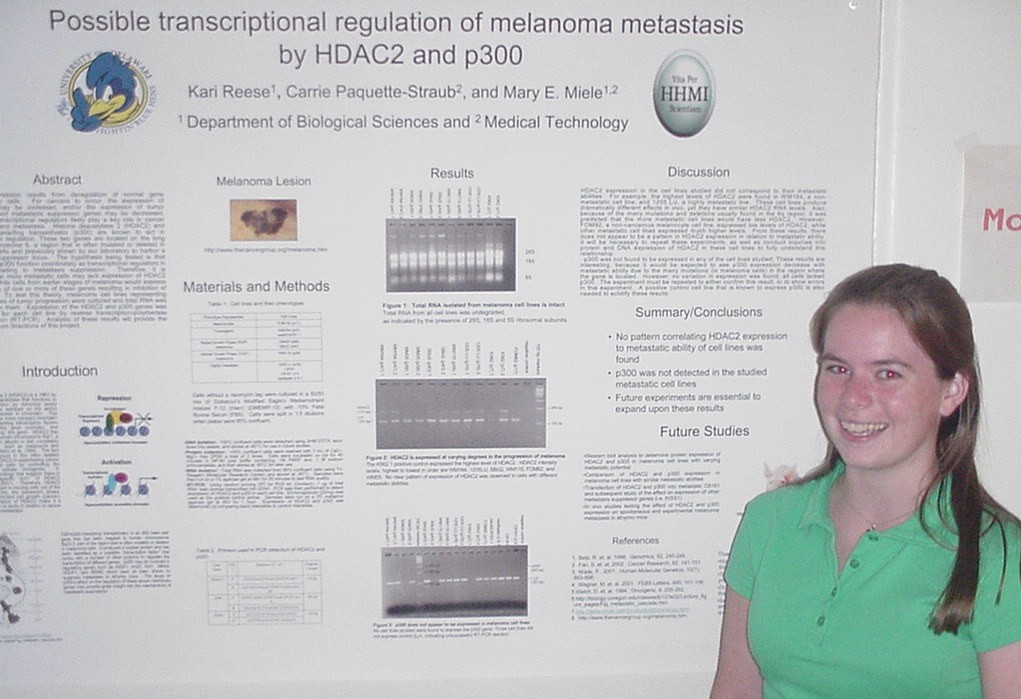
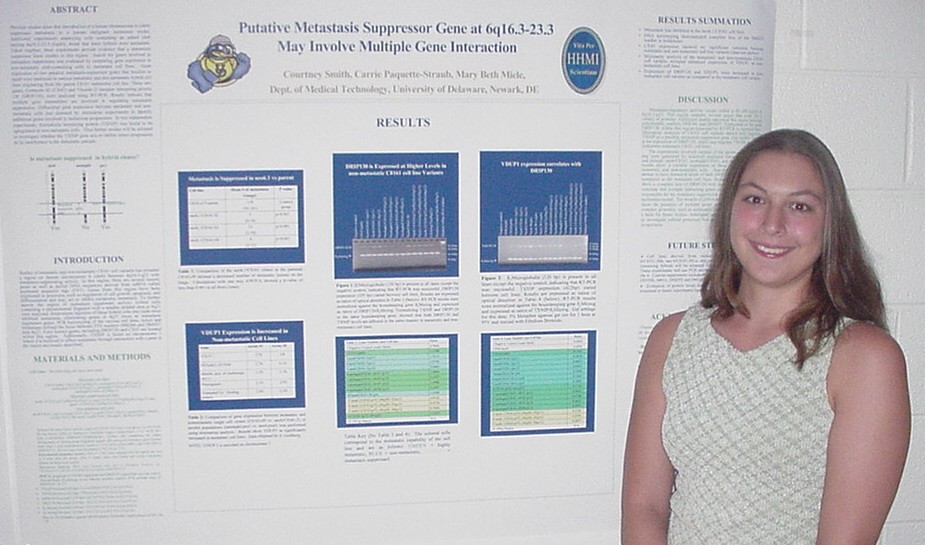
of Metastasis Suppressor Genes
Sarah Baisch, Carrie Paquette-Straub, and Mary E. Miele
Department of Medical Technology
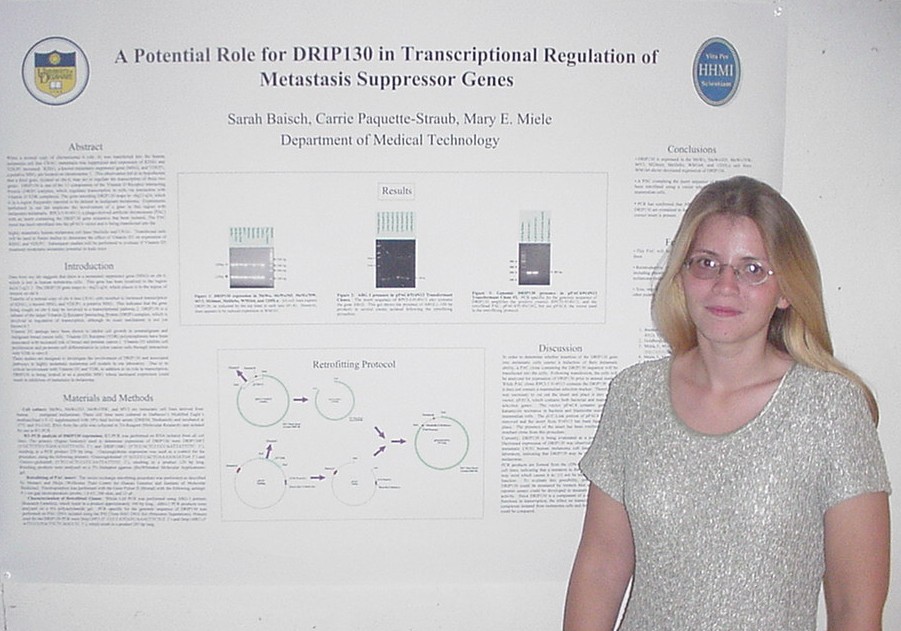
When a normal copy of chromosome 6 (chr. 6) was transferred into the
human melanoma cell line C8161, metastasis was suppressed and expression
of KISS1 and VDUP1 increased. KISS1, a known metastasis suppressor gene
(MSG), and VDUP1, a putative MSG, are located on chr.1. This observation
led us to hypothesize that a third gene, located on chr.6, may regulate
the transcription of these two genes. DRIP130 is one of the 13 components
of the Vitamin D Receptor Interacting Protein (DRIP) complex, which regulates
transcription in cells via interaction with Vitamin D-VDR complexes. The
gene encoding DRIP130 maps to ~6q22-q24, which is in a region frequently
reported to be deleted in malignant melanoma. Experiments performed in
our lab implicate the involvement of a gene in this region with melanoma
metastasis. RPCI-5-914N13, a phage-derived artificial chromosome (PAC)
with an insert containing the DRIP130 gene sequence, has been isolated.
The PAC insert has been retrofitted into the pPAC4 vector and is being
transfected into the highly metastatic human melanoma cell lines MelJuSo
and C8161. Transfected cells will be used in future studies to determine
the effect of Vitamin D3 on expression of KISS1 and VDUP1. Subsequent studies
will be performed to evaluate if Vitamin D3 treatment modulates metastatic
potential in nude mice.