Gregory G. Abendroth, Matthew J. Gage, and Anne Skaja Robinson
Department of Chemical Engineering
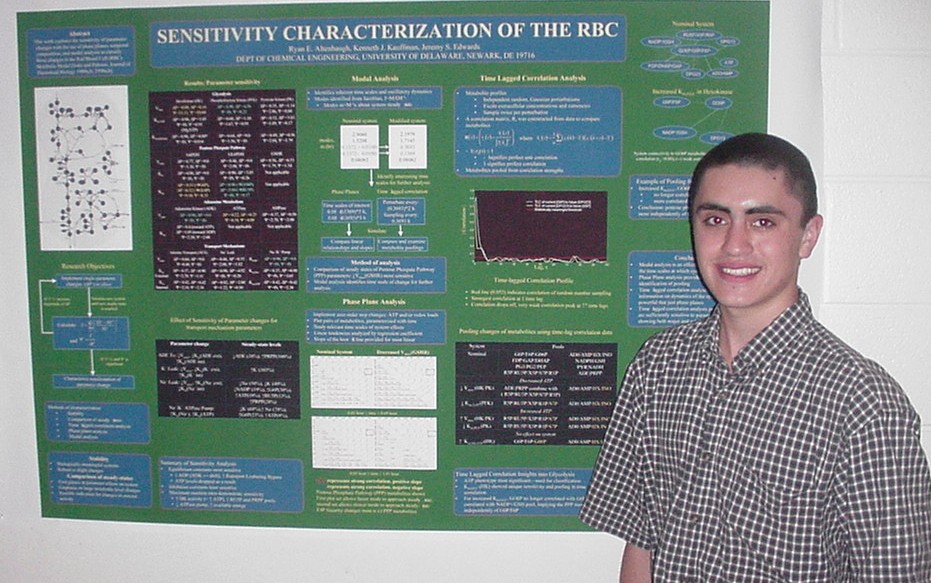
Correct folding is critical for protein function, and aggregates resulting from misfolding cause numerous human diseases. The insoluble nature of protein aggregates necessitates the use of model systems, such as the P22 tailspike, for examining the factors that affect aggregation. P22 tailspike is a protein comprised primarily of beta strands in a beta-helix motif. The role of histidine 420 in P22 tailspike was examined by mutating H420 to phenylalanine (H420F) using Stratagene’s QuikChange Site-Directed Mutagenesis Kit. This mutant was chosen for study to examine the effect of charge on folding of the beta helix domain. The H420F mutant formed a trimer during expression in E. coli and was purified by anion exchange and hydrophobic affinity column chromatography. Chemical denaturation studies indicate that H420F may be more stable than wild-type tailspike. However, thermal denaturation suggests it may be less stable. Preliminary in vitro refolding studies indicate it may not form trimer at neutral pH. Additional experimentation is underway to help clarify the folding and stability of H420F.