Background for the article by
C. G. Douglas, J. S. Haldane, and J. B. S. Haldane (1912)
The Laws of Combination of Hæmoglobin
with Carbon Monoxide and Oxygen
Background
What is not clear at the time is often so clear to us in retrospect. Frequently it takes many years and many experimental confirmations to convert experimental facts in to accepted knowledge. By demonstrating a definite stoichiometry between iron of the hematin and the sulfur of the protein (globin) portions of hemoglobin, Zinoffsky (1) showed clearly that hemoglobin was, in fact, a discrete complex between the relatively small hematin (4% by weight) and the much larger protein whose molecular weight he could estimate. Other researchers performed similar analyses on hemoglobin from a variety of species and came to similar conclusions. Nevertheless, the techniques available to nineteenth century chemists did not deal effectively with the structures of such an enormous molecule as hemoglobin let alone the much smaller heme prosthetic group. While the elemental composition of hematin was known accurately in the early 1900's and some indications of its structure were postulated in 1913, the accepted complete structure was not proven until 1930 (2). The protein portion also remained elusive and some perhaps hoped it was not really so large as Zinoffsky's data suggested. Gustav Mann (1906), in his book Chemistry of the Proteids (3), gave the minimum molecular weight of hemoglobin as 16,669 but states (p 324), "The molecular weight of globin, i.e. the albumin-radical of hemoglobin, may of course be very much less, as we do not know whether the colouring radical, the hæmatin, is joined up with one or several molecules of globin." He then expressed frustration that the direct methods for determining molecular weights such as melting point elevation, freezing point depression, and osmotic pressure, had not worked for globulins.
Zinoffsky (1) also expressed frustration with the available methods. He wanted to determine, "the amount of loosely bound oxygen in order to decide if this element also has a simple stoichiometric relationship to iron." Near the turn of the century methods were developed to release oxygen stoichiometrically from oxyhemoglobin and to collect and measure it. J. S. Haldane (4) showed that the reaction of oxyhemoglobin with ferricyanide rapidly and quantitatively released oxygen and formed methemoglobin. The liberated oxygen could be accurately calculated from the increased gas pressure in the closed reaction system at constant temperature and volume. Your reader includes and article by Barcroft and Haldane (5) which describes the apparatus and method for measuring oxygen and carbon dioxide in small amounts of blood. In 1912, Peters (6) used this apparatus to show decisively, as others had concluded and perhaps Zinoffsky had suspected, that there was a 1 to 1 stoichiometry between loosely bound oxygen and iron. (Your reader also contains the article by Sir Rudolph Peters.) While stoichiometry is important, it does not reveal the nature of the chemical association. As Mann states (2, p 490), "At the present time [1906] it is impossible to make any definite statement as to how oxygen is united with the hemoglobin molecule, but it is generally assumed that the power of absorbing oxygen is dependent on the iron or the iron-containing radical hæmatin."
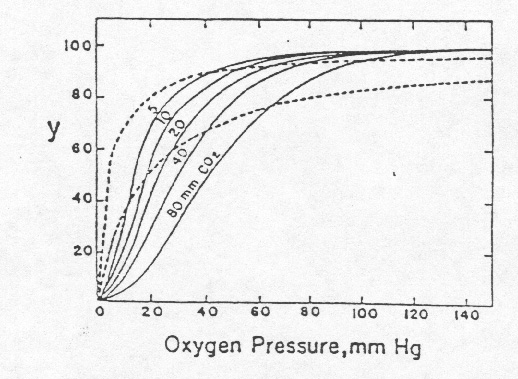
Oxygen is so important to animal life. Little wonder that Haldane, Peters, and many other prominent physiologists devoted much of their careers to studying hemoglobin and its role in oxygen transport and that many of the major advances in the biochemistry of proteins were first made with hemoglobin (7-9). Despite the careful analytical work of Zinoffsky, Peters, and others, there were still unexplained and perplexing phenomena observed with hemoglobin. For example, if one assumes that the binding of one mole of oxygen with one mole of hemoglobin were a simple association-dissociation equilibrium, the amount of bound oxygen should be a simple function of the concentration (partial pressure) of oxygen. Bohr, Hasselbalch, and Krogh (10) (in your reader), working with dog blood, obtained the results in the figure below where oxygen binding showed a sigmoidal relationship to oxygen pressure and carbon dioxide had, as puzzled Stokes (11), a considerable effect on the binding of oxygen. Several scientists, notably Archibald V. Hill (12), modified the simple equilibrium binding model to account for the sigmoid relationship.

Fig. 1 The solid lines represent the data of Bohr, Hasselbalch, and Krogh, for binding of oxygen by hemoglobin in dog blood, at various partial pressures of CO2. The two dashed lines are rectangular hyperbolas, showing binding curves that would correspond to the equation, y = kp/(1+kp); the hyperbolic curve on the left is for half saturation with oxygen at 4 mm Hg oxygen pressure (K = 0.25 mm-1), the curve on the right is for half saturation at 20 mm (K = 0.05 mm-1). The ordinate, y, denotes percentage saturation with oxygen. [Figure and legend taken from Edsall (7).]
While carbon dioxide affected oxygen binding, it did not directly compete with oxygen. However carbon monoxide did compete with oxygen and actually bound 300 times more tightly than oxygen, a phenomena of considerable importance to the safety of coal miners in Great Britain. J. S. Haldane thus studied the interactions of carbon monoxide and oxygen in binding to hemoglobin with great interest not only because of its fundamental interest but also because of its direct importance to the welfare of fellow citizens. When you read the next article, be aware that it is not just some esoteric and irrelevant curiosity.
About the Haldanes
J(ohn) S(cott) Haldane (1860 - 1936), Fellow of the Royal Society, Professor at Oxford, and once President of the Society of Mining Engineers, often experimented on himself. He, for example, deliberately subjected himself to dangerous but sub lethal concentrations of carbon monoxide to experience the effects (13). He suggested the use of canaries in mines as detectors of carbon monoxide accumulation. In 1911, he and several other physiologists organized an expedition to the top of Pikes Peak in Colorado to study the effects of low oxygen at high altitudes (14, 15). Despite his many contributions to physiology and his use of chemical and physical techniques, he opposed a reductionist approach to biology. He believed that organisms obeyed special laws different in principle from those of chemistry and physics. He once wrote, "Those who aim at physico-chemical explanations of life are simply running their heads into a stone wall, and can only expect sore heads as a consequence (16)." His brother was Secretary of State for War for Great Britain from 1914 - 1918 during the First World War.
J(ohn) B(urdon) S(anderson) Haldane (1892 - 1964), a member of the British, American, German, Soviet, and Danish national science academies, began his scientific career at the age of 8 as an assistant in his father's laboratory. His very first scientific article was published when he was only 19 and a second year college student. After studying humanities at Oxford and serving with the Black Watch on the front lines in France, he returned to science as a lecturer in biochemistry at Cambridge University. Like his father he was noted for self-experimentation. In contrast to his father, he had no reservations about reductionist approaches to biology. He, independently of Oparin in Russia, published an insightful article on the origin of life (17). He authored influential books on a number of subjects including enzymes (18), evolution (19), and biochemical genetics (20). His political views put him at odds with the British government. In the 1930's he was an editor of the London Daily Worker, a communist newspaper, and he aided refuges from Germany. In 1957, he moved to India in self-imposed exile (21).
References
2. Fischer, H (1930) (Fischer received the 1930 Nobel Prize in Chemistry)
3. Mann, G. (1906) Chemistry of the Proteids, Macmillan and Co., Ltd., London. (More than 10% of this 584 page book is devoted to hemoglobin and hematin.)
4. Haldane, J. S. (1898) A Contribution to the Chemistry of Hämoglobin and its Immediate Derivatives, J. Physiol. 22, 298 - 306. (See also J. Physiol. 25, 295 - 302 (1900)).
5.* Barcroft, J. and Haldane, J. S. (1902) A Method of Estimating the Oxygen and Carbonic Acid in Small Quantities of Blood, J. Physiol. 28, 232 - 240. (Further refinements of this gas-measuring apparatus became the Warburg Respirometer which was very important in studying metabolism into the 1960's and is still used in some laboratories.)
6.* Peters, R. A. (1912) Chemical Nature of Specific Oxygen Capacity in Hämoglobin, J. Physiol. 44, 131 - 149.
7. Edsall, J. T. (1972) Blood and Hemoglobin: The Evolution of Knowledge of Functional Adaptation in a Biochemical System. Part I: The Adaptation of Chemical Structure to Function in Hemoglobin, J. Hist. Biol. 5, 205 - 257.
8. Edsall, J. T. (1980) Hemoglobin and the Origin of the Concept of Allosterism, Fed. Proc. 39, 226 - 235.
9. Edsall, J. T. (1986) Understanding Blood and Hemoglobin: An Example of International Relations in Science, Persp. Biol. Med. 29, S107 - S123.
10. Bohr, C., Hasselbalch, K. A., and Krogh, A. (1904) Über Einen in Biologischen Beziehung wichtigen Einfluss, den die Kohlensäurespannung des Blutes auf desen Sauerstoffbinding Übt. Skand. Arch. Physiol. 16, 401 412. (A translation is in your reader.) (Bohr's son Niels received the 1922 Nobel Prize in Physics. Hasselbalch's name should be familiar from the Henderson- Hasselbalch Equation relating pH and pKa with the concentrations of acids and conjugate bases. Krogh became a famous physiologist.)
11.* Stokes, G. G. (1864) On the Reduction and Oxidation of the Colouring Matter of the Blood, Proc. Royal Soc. London 13, 355 - 364.
12. Hill, A. V. (1913) The Combinations of Hæmoglobin with Oxygen and Carbon Monoxide, Biochem. J. 7, 471 - 480. (Although Hill's physical model of oxygen binding later proved to be incorrect, his mathematical model, The Hill Equation, is still widely used in biochemistry for systems involving cooperative interactions.)
13. Haldane, J. S. (1895) The Relation of the Action of Carbonic Oxide to Oxygen Tension, J. Physiol. 18, 201 - 217.
14. Douglas, C. G., Haldane, J. S., Henderson, Y. et al. (1913) Physiological Observations made on Pike's Peak, Colorado with Special Reference to Adaptation to Low Barometric Pressure, Phil. Trans. Royal Soc. B 203, 185 - 318.
15. Milledge, J. S. (1985) The Great Oxygen Secretion Controversy, The Lancet Dec 21/28, 1408 - 1411. (This article contains pictures of August Krogh, J. S. Haldane, and Joseph Barcroft.)
16. Haldane, J. S. (1908) The Relation of Physiology to Physics and Chemistry, Nature 78, 553 - 556.
17. Haldane, J. B. S. (1928) The Origin of Life, Rationalist Annual 148, 3 - 10. (Reprinted in 1985 in On Being the Right Size, and Other Essays, (J Maynard Smith, ed. ) pp 101 - 112, Oxford University Press.)
18. Haldane, J. B. S. (1930) Enzymes, Longmans, Green and Co., Ltd, Great Britain. (Reprinted in 1965 by the Massachusetts Institute of Technology.)
19. Haldane, J. B. S. (1932) The Causes of Evolution, London.
20. Haldane, J. B. S. (1954) The Biochemistry of Genetics, George Allen & Unwin, Ltd., London. (this book was published shortly after Watson and Crick's famous article on the structure of DNA and thus represents perhaps the last "pre-DNA" summary of biochemical genetics.)
21. Clark, R. W. (1968) JBS: The Life and Work of J. B. S. Haldane, Coward-McCann Inc. New York.
* Articles in your course reader.