![[portrait]](bulkowski.jpg)
Associate Professor, Inorganic Chemistry
Phone: (302)831-2467
Email: jbulkow@udel.edu
![[portrait]](bulkowski.jpg) |
Associate Professor, Inorganic Chemistry Phone: (302)831-2467 Email: jbulkow@udel.edu |
Sc.B., Brown University, 1965
Ph.D., Carnegie-Mellon University, 1973
Postdoctoral, Harvard University, 1974-1975
CURRENT RESEARCH
One area of my research involves binuclear transition metal coordination complexes as biomodels and homogeneous catalysts. The focus is the design of molecular systems to activate small molecules such as dioxygen at two metal reaction centers.
Our approach uses the ligand frameworks of hexaamine macrocycles to control intermetal separations and maintain the structural integrity of the binuclear unit. We have developed synthetic routes to mono- and bicyclic macrocycles and demonstrated they form stable bimetallic complexes. X-ray studies of the metal complexes show their propensity for the formation of unusual bridged derivatives with small molecules. For example, we have isolated and structurally characterized the first example of a dioxygen-bridged dicopper complex. Studies of oxygen binding in these systems provides an opportunity for understanding dioxygen activation at bimetallic metalloprotein active sites and in homogeneous oxidation catalysts. A related series of dicopper(II) monohydroxo-bridged complexes have also been isolated and structurally characterized. Discrete oxo-bridged dicopper(II) species prepared from these precursors are being used to study the reactivity and redox properties of the oxo-bridged dicopper(II) unit. The mono-hydroxo species are also readily converted to dihydroxo complexes which have shown a unique reactivity with small molecules such as nitromethane.
Another aspect of this work focuses on the chemistry of dicopper(I) complexes of these macrocycles as selective oxidation catalysts for the conversion of phenols to benzoquinones in the presence of molecular oxygen. Mechanistic studies suggest a catalytic cycle for the oxidation process employing the binuclear metal site for both dioxygen activation and selectivity. We are exploring the use of this system for the catalytic oxidation of other aromatics and olefins.
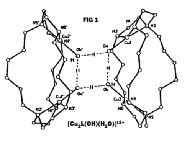 In our work with bicyclic ligands, we have isolated a tetranuclear
copper(II) dimer in which four water molecules are spontaneously self-assembled
via hydrogen-bonding interactions between the four metal centers (Figure
1). This and related manganese complexes could model multimanganese active
sites relevant to the dioxygen-generating process in green plant photosynthesis.
In our work with bicyclic ligands, we have isolated a tetranuclear
copper(II) dimer in which four water molecules are spontaneously self-assembled
via hydrogen-bonding interactions between the four metal centers (Figure
1). This and related manganese complexes could model multimanganese active
sites relevant to the dioxygen-generating process in green plant photosynthesis.
Another area of my research is the synthesis and characterization of
solid polymeric materials exhibiting high ionic conductivities at room
temperature for potential application in thin film batteries, sensors and
microelectronic devices. The materials consist of metal-doped organic copolymers
which incorporate macrocycles con-taining nitrogen and oxygen metal ion
binding sites directly into the polymer backbone. Ionic conductivity studies
of unpolymerized macrocycles in the solid state show the lithium ion doped
materials have appreciable conductivities. The conductivity was found to
be great-ly dependent on the level of doping and the size and flexibility
of the macrocycle. Detailed studies of the ionic conductivity in both the
free macrocycles and macrocycle-containing copolymers offer exciting prospects
for understanding the mechanism of ion transport in solid polymers and
may result in the development of a new class of solvent free ion-conducting
polymeric materials.
REPRESENTATIVE PUBLICATIONS